Journal of Medical Diagnostic Methods
Open Access
ISSN: 2168-9784
ISSN: 2168-9784
Research Article - (2023)Volume 12, Issue 5
The clinical significance of Anti-Sperm Antibodies (ASAs) was re-evaluated by comparing motile sperm without DNA fragmentation (Live Sperm: LS) and immotile sperm with end-stage DNA fragmentation (Denatured Sperm: DS). Immunoglobin G was partially purified from the sera of women of infertile couples, and the location of antigenic sites on LS and DS were observed via Indirect Immunofluorescence Staining (IIFS). LS and DS corresponded to sperm that had not yet and those that had already undergone apoptosis, respectively. IIFS suggested that ASAs were produced frequently for the acrosome cap, the equatorial segment, a point-like organelle at the junction of the head/midpiece, the midpiece, the junction of the midpiece/principal piece of the tail, the principal piece of the tail, and the terminal piece of the tail. DS degraded the binding capacity towards all observed ASAs.
Human sperm; Anti-sperm antibody; Apoptosis; DNA fragmentation; Immune infertility; Indirect immunofluorescence staining; One-dimensional single-cell pulsed-field gel electrophoresis
Sperm intruding into the peritoneal cavity through the fimbriae of the fallopian tube are recognized by the body as having foreign antigens; in response, anti-sperm alloantibodies are produced through macrophage phagocytosis and presentation to the T cells [1]. The blood-testis and blood-epididymis barriers prevent exposure of the germ cells to the circulating immunocompetent cells, and failure of these barriers allows the production of antisperm autoantibodies [2,3].
To date, sperm immobilization tests [4], sperm agglutination tests [5], immunobead (IB) [5,6], tests and mixed anti-globulin reaction (MAR) tests [6,7], have been used to detect anti-sperm antibodies (ASAs). Many literatures accept that binding of ASAs to spermatic surface antigens impairs sperm function [8,9], adversely affects the sperm-egg interaction [10], and results in phagocytosis of the sperm in the female reproductive tract [11]. The incidence of immune infertility is 10%–30% among infertile couples, [12,13], and 40–70% of vasectomized men develop ASAs [14,15].
We previously revealed that human sperm was heterogeneous population in terms of not only motility and DNA integrity, and purified human motile sperm without DNA fragmentation and immotile sperm with end-stage DNA fragmentation for use as comparative standards in DNA fragmentation analyses [16]. Furthermore, comparison of these standards suggested that the antigenicity of human sperm diminished during DNA fragmentation.
In terms of the significance of ASAs in clinical immune infertility, this result warrants investigation of ASAs with motile sperm without DNA fragmentation, as this interaction affects fertility. The present study was conducted to re-evaluate analytical methods and diagnostic criteria of ASAs in immune infertility by means of indirect immunofluorescence staining.
Ethics statement
Ejaculates were obtained from volunteers. In the present study, we examined ASAs in the sera of 77 individuals, of which 67 were from the women in infertile couples and 10 from women who became pregnant spontaneously and visited our infertility clinic or obstetrics department, respectively. The aim of this study and the measurement items were clearly explained to them, and they provided written informed consent for participation in and publication of the study. The ethics committee of the Ichikawa General Hospital approved this study (approval no. 2013-02).
Purification of human sperm without DNA fragmentation or with granular segments in end-stage fragmentation
Sperm concentration and motility were determined according to the WHO reference manual [17]. Human motile sperm without DNA fragmentation and immotile sperm with end-stage fragmentation were purified according to the protocol presented in our previous report [16]. Briefly, the sperm were fractionated by means of sedimentation equilibrium in a discontinuous OptiPrep (OP, apparent density of 1.085 and 1.17 g/mL; Axis Shield, San Jose, CA, USA) gradient and subsequent differential velocity sedimentation in an isotonic 90% Percoll (GE Healthcare, Chicago, IL, USA) density gradient. Progressively motile sperm were recovered in the interface layer of the OP/sediment of Percoll. Immotile sperm with granular DNA segments were recovered in the sediment of OP/intermediate layer of Percoll.
One-dimensional Single-Cell Pulsed-Field Gel Electrophoresis (1D-SCPFGE)
Single-cell nuclear DNA fragmentation was measured with 1D-SCPFGE according to the protocol presented in previous reports [16,18,19]. In brief, sperm were embedded in molten 0.5% agarose containing 20 μg/mL highly purified bovine pancreatic trypsin (50 mmol/L acetate buffer, pH 4.7) to form a 100 μmthick gel coating. The gel film was incubated in cell lysis reagent (50 mmol/L Tris-HCl, 8.2 mmol/L sodium hexametaphosphate, 0.05% Triton X-100, and 5.0 mmol/L dithiothreitol, pH 8.2) at 37°C for 30 min. The apparatus was equipped with dual electrode pairs arranged at a 60° angle and electrophoresis was performed at 2.5 V/cm with 4.0-s switching intervals for 7 min in electrophoresis buffer (30 mmol/L Tris-acetate, pH 8.2). The DNA in the gel was stained with diluted (× 104) SYBR Gold stain (Molecular Probes, Eugene, OR, USA) and observed under an epifluorescence microscope with a green filter (Axio Imager A1; Carl Zeiss MicroImaging, Jena, Germany). Still images were recorded using an AxioCam HRM camera (Carl Zeiss). Sperm with a bundle of elongated long-chain fibers from the origin without any fibrous segments beyond the anterior end of the elongated fibers were classified as intact, and those with at least one fibrous fragment were classified as damaged.
Indirect immunofluorescence staining
Immunoglobulin G (IgG) was partially purified from the sera by means of ion-exchange absorption with DEAE Sephadex A50 [20]. The swollen DEAE Sephadex in pure water (0.45 mL bed volume) and mixed with 0.05 mL of the serum in a centrifugal filter unit (Ultrafree-MC-HV, Merk Millipore, Barlington, MA, USA). The filtrate (0.27 mL) was made isotonic with 0.03 mL of 200 mmol/L HEPES-buffered, 10 × Hanks’ solution.
An aliquot of purified sperm (106/slide) were adhered to a plane glass slide with the aid of centrifugal auto-smear (Cyto-Tek; SAKURA, Tokyo, Japan). The partially purified IgG fraction (0.3 mL, hereafter referred to as “IgG”) was mounted on the sperm and incubated for 30 min at room temperature. After two washes with saline, an aliquot (200 μL) of Alexa 488-conjugated goat anti-human IgG (2.0 μg/mL in Hanks’ solution, Molecular Probes, Eugene, OR, USA) was mounted on the sperm and incubated for 30 min at room temperature. The slide was washed with saline and observed under a fluorescence microscope with a green filter (Axio Imager A1), and still images were recorded using the AxioCam HRM camera.
Observation of vacuoles in the head of sperm
The sperm (106/slide) was adhered to a plane glass slide by using centrifugal auto-smear and fixed with methanol for 5 min. The specimens were stained with 0.02% reactive blue 2 (RB2; 0.1 mol/L Na2CO3-NaHCO3, pH 10.0) for 10 min, and the excess dye was washed out with pure water [21]. The images were observed under oil immersion by using an upright, transmitted brightfield light microscope (Axio Imager A1; × 1000 magnification). Still images were recorded using an AxioCam HRC camera.
Differential observation of plasma and inner acrosomal membranes via two-step concanavalin-A labeling
Concanavalin A has high affinity to the high-mannose sugar chains on the inner acrosomal membrane [22]. Sperm suspension (106/slide) was incubated with an equal volume of isotonic Cy3- conjugated concanavalin A (Molecular Probes) at 37°C for 20 min. Thereafter, the reaction mixture was adhered to a plane glass slide via centrifugal auto-smear, treated with methanol to exclude the plasma and the outer acrosomal membranes, and mounted with 0.2 mL of Alexa 488-conjugated concanavalin A (Molecular Probes) at ambient temperature for 20 min. The slide was washed out with saline, and the same field of view was observed under a fluorescence microscope with a combination of green and red filters (Axio Imager A1; × 1000 magnification). Still images were recorded using the Axio Cam HRC camera and merged with the aid of Image J software (version. 1.5.3). Red fluorescence on the merged photograph indicated that the plasma and the outer acrosomal membranes had already been damaged; facilitating the permeation of Cy3-conjugated concanavalin A. Green fluorescence indicated that the inner acrosomal membrane was exposed after methanol treatment and bound with Alexa 488-conjugated concanavalin A, suggesting that the acrosome was intact.
Almost the sperm recovered from the interface layer of the OptiPrep/sediment of Percoll was progressively motile (Video 1A). The apparent density was estimated as in the range of 1.12- 1.17 g/mL [16]. 1D-SCPFGE revealed that most sperm had a bundle of elongated long-chain fibers from the origin (Figure 1A). The sperm fraction with more than 80% motility and more than 80% and the negative rate of DNA fragmentation more than 80% was termed “Live Sperm” (LS) and used as the test object for Indirect Immunofluorescence Staining (IIFS).
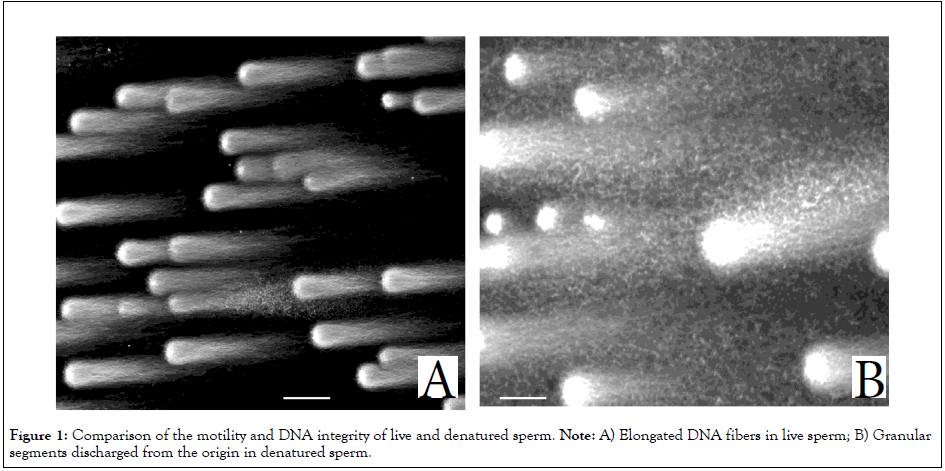
Figure 1: Comparison of the motility and DNA integrity of live and denatured sperm. Note: A) Elongated DNA fibers in live sperm; B) Granular segments discharged from the origin in denatured sperm.
Video 1A: Comparison of the motility and DNA integrity of live and denatured sperm, Video of Live sperm.
Note: Video 1A is recorded at 200X magnifications.
In contrast, sperm recovered from the sediment of the OptiPrep/ intermediate layer of Percoll were almost immotile and highly auto-agglutinated (Video 1B), the apparent density was estimated to exceed 1.17 g/mL [16]. Almost these sperm discharged granular segments from the origin (Figure 1B), suggesting that they had already entered end-stage fragmentation. These were termed “Denatured Sperm” (DS) (Figure 1).
Video 1B: Comparison of the motility and DNA integrity of live and denatured sperm, Video of Denatured sperm.
Note: Video 1B is recorded at 200X magnifications.
The features of the acrosome and the vacuoles in the head were compared between LS and DS (Figure 2). Most of the acrosome in LS exhibited green fluorescence (Figure 2A), suggesting that the plasma and the outer acrosomal membranes were intact until methanol treatment. In contrast, most of those in DS exhibited red fluorescence (Figure 2B), suggesting that the damaged plasma and outer acrosomal membranes facilitated permeation of concanavalin A into the inner acrosomal membrane. The feature of the vacuoles did not differ between LS and DS (Figures 2C and 2D).
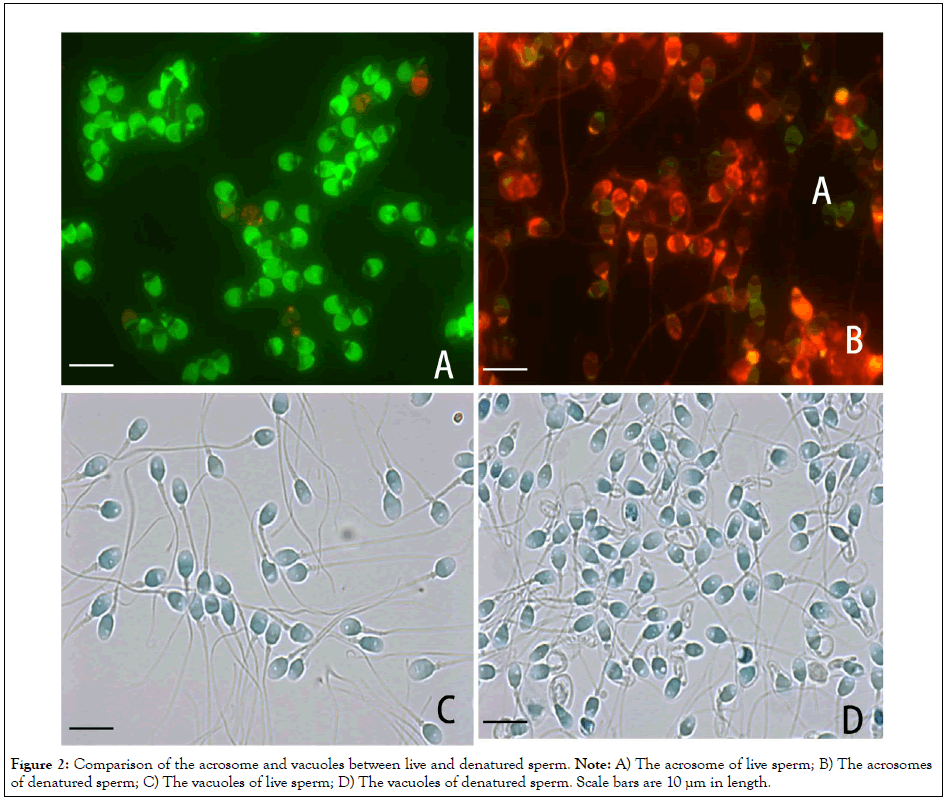
Figure 2: Comparison of the acrosome and vacuoles between live and denatured sperm. Note: A) The acrosome of live sperm; B) The acrosomes of denatured sperm; C) The vacuoles of live sperm; D) The vacuoles of denatured sperm. Scale bars are 10 µm in length.
The titer is used as the index of antibody valency in immunochemical analyses. Sperm that enter the female genital tract are exposed to a large excess of IgGs in the bodily fluids. Thus, LS must preferably be mixed with the undiluted serum to evaluate the significance of ASAs in clinical immune infertility. Mammalian serum is well-known to promote the acrosome reaction facilitating exposure of the inner acrosomal membrane [23]. In the present study, we uniformly mixed LS with partially purified IgGs from the sera corresponding to approximately 10 × diluted serum. When LS were incubated with the IgG fraction of ASA-negative serum at 37°C for 20 min, green fluorescence was observed, similar to that shown in Figure 2A, suggesting that their inner acrosomal membrane was not exposed (data not shown).
Figures 3 and 4 summarized typical binding sites of ASAs on LS; sperm in images with no or minimal fluorescence were classified as negative for ASAs (Figure 3A). ASAs were frequently produced against the acrosome cap (Figure 3B), the equatorial segment (Figure 3C), the point-like organelle at the junction of the head/ midpiece (Figure 3D), the midpiece (Figure 4A), the junction of the midpiece/ principal piece of the tail (Figure 4B), the principal piece of the tail (Figure 4C) and the terminal piece of the tail (Figure 4D). ASAs are naturally produced polyclonal antibodies, and the sera often contained heterogeneous IgGs binding to more than one antigenic site on LS (Figure 3B). Of 67 IgG fractions in the women in infertile couples, 23 specimens exhibited at least one antigenic site classified as positive. Of 36 positive sites, the most common sites were the equatorial segment (7 sites), the principal piece of the tail (5 sites), the junction of the head/ midpiece (4 sites), and the midpiece (3 sites). In the present study, we also observed 10 women who became pregnant spontaneously, 4 of whom were positive for ASAs in the sera that were submitted for pregnancy testing. ASAs bound to the equatorial segment (2 cases), a point-like organelle at the junction of the head/midpiece (1 case), and the principal piece of the tail (1 case).
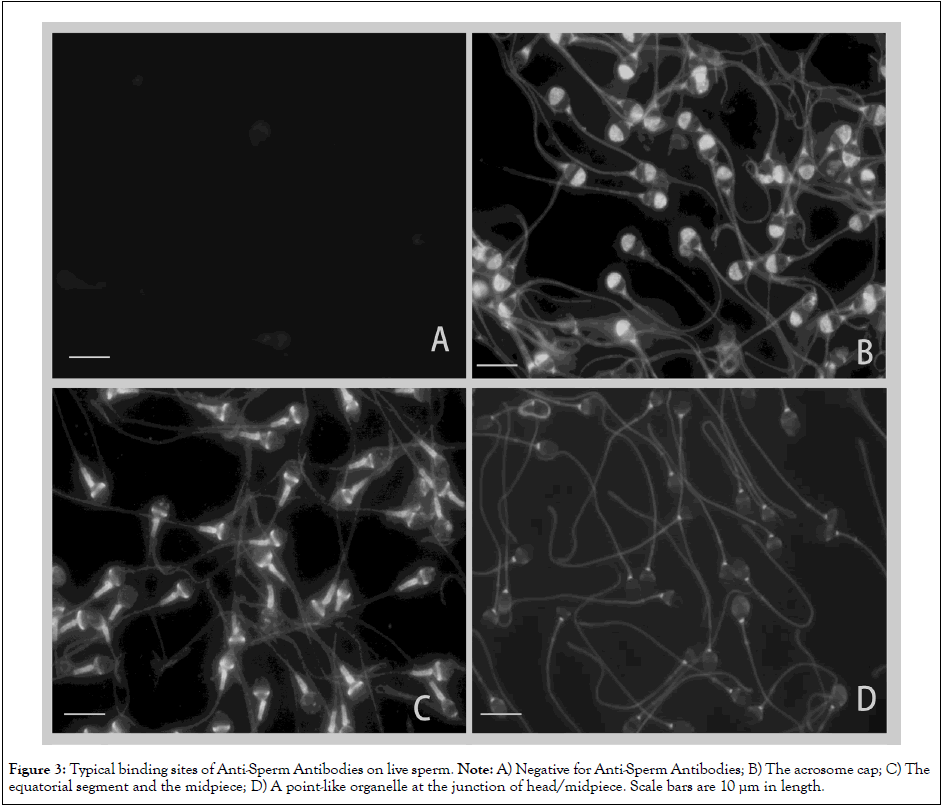
Figure 3: Typical binding sites of Anti-Sperm Antibodies on live sperm. Note: A) Negative for Anti-Sperm Antibodies; B) The acrosome cap; C) The equatorial segment and the midpiece; D) A point-like organelle at the junction of head/midpiece. Scale bars are 10 µm in length.
Video 2 shows head-to-head auto-agglutination of LS via the IgGs of a woman in an infertile couple; the cluster of sperms moved vigorously. IIFS suggests that ASAs binding to the equatorial segment connected the heads of sperm in a side-by-side manner (Figure 5). The same IgGs did not fluoresce at this site on DS (Figure 6), which entangled with one another non-specifically and formed static clusters (Figure 7). This feature was similar to that shown in Video 1B.
To know whether disappearance of the antigenic site in DS was limited to the equatorial segment (Figure 6), the IgGs shown in Figures 3 and 4 were mixed with DS. As displayed in Figure 8, the antigenicity of all the sites, including the acrosome (Figure 8A, corresponds to Figure 3B), a point-like organelle at the junction of the head/midpiece (Figure 8B, corresponds to Figure 3D), the midpiece (Figure 8C, corresponds to Figure 4A), and the principal piece of the tail (Figure 8D, corresponds to Figure 4C), was uniformly degraded. This suggests that the antigenic molecules are absent from the sperm surface rather than that the antigenicity of the DS is altered.
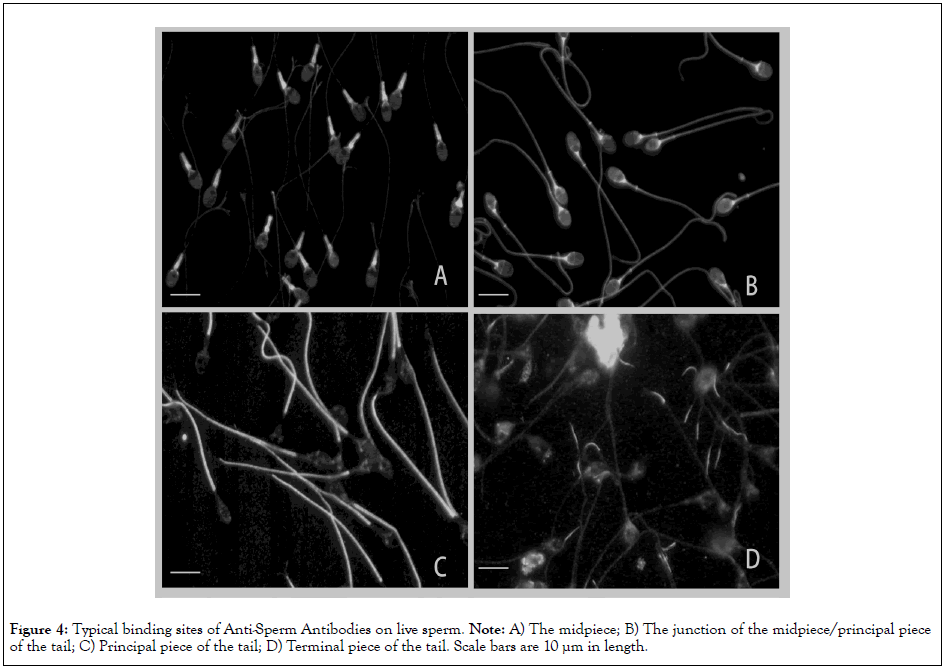
Figure 4: Typical binding sites of Anti-Sperm Antibodies on live sperm. Note: A) The midpiece; B) The junction of the midpiece/principal piece of the tail; C) Principal piece of the tail; D) Terminal piece of the tail. Scale bars are 10 µm in length.
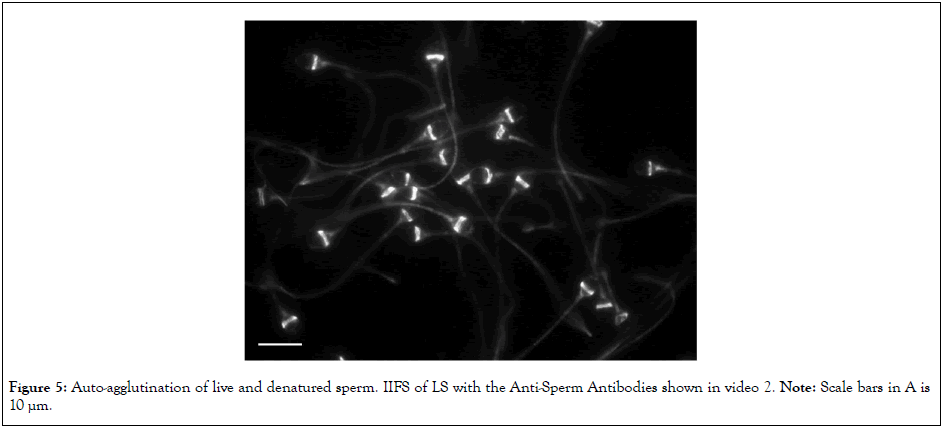
Figure 5: Auto-agglutination of live and denatured sperm. IIFS of LS with the Anti-Sperm Antibodies shown in video 2. Note: Scale bars in A is 10 µm.

Figure 6: Auto-agglutination of live and denatured sperm. IIFS of denatured sperm with the Anti-Sperm Antibodies shown in video 2 Note: Scale bars in B is 10 µm.
Apoptosis plays a significant role not only in spermatogenetic quality control but also in sperm denaturation [24,25]. Release of cytochrome C from the mitochondria, an initiating step in apoptosis, interferes with sperm motility [26]. Cell shrinkage via apoptotic volume decrease results in an increased apparent density [27]. DNA fragmentation [16,18,19] is the most common feature of apoptosis. Denaturation of the plasma membrane also occurs in apoptosis [28]. DS allowed permeation and binding of Cy3- conjugated concanavalin A to the inner acrosomal membrane (Figure 2B), which results in cluster formation through autoagglutination (Video 1B and Figure 7). The features shown in Figures 1 and 2 as well as our previous data [16] strongly suggest that LS and DS correspond to sperm that have not yet and those that have already undergone apoptosis, respectively. Apoptosis generally facilitates antigen presentation of somatic cells to T lymphocytes [29]. In natural intercourse, the motile LS enter the cervix and reach the peritoneal cavity, where they present their antigens, whereas the immotile DS stay in the vagina, where they do not encounter the abdominal immune cells. Disulfide cross-linking of protamines is essential for the condensation and organization of the shape of the mammalian sperm head [30]. Our previous study suggested that incompetent cross-linkage among protamines is closely related to vacuole formation [21]. The present study suggests that apoptosis does not affect the of vacuoles (Figures 2C and 2D).

Figure 7: Auto-agglutination of live and denatured sperm. Non-specific auto-agglutination of denatured sperm, forming a static cluster. Note: Scale bars in C is 50 µm in length.
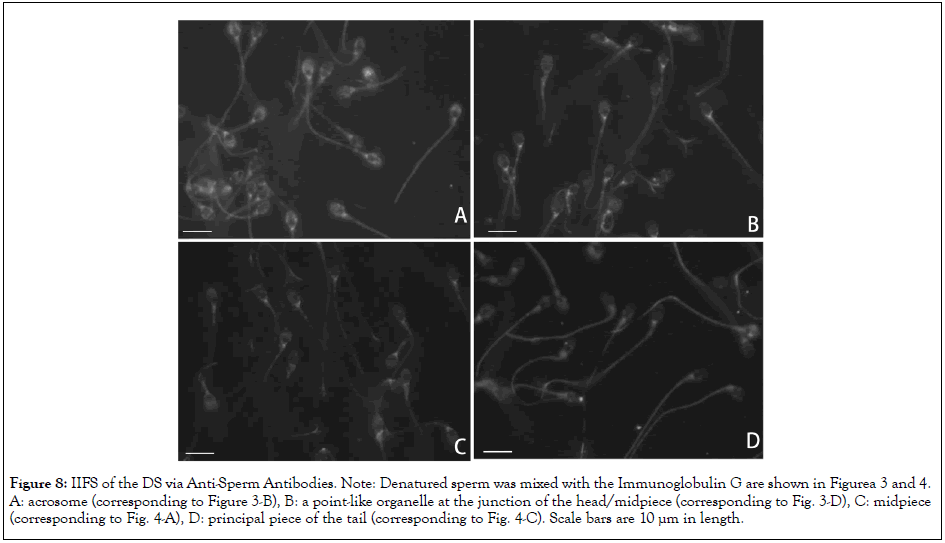
Figure 8: IIFS of the DS via Anti-Sperm Antibodies. Note: Denatured sperm was mixed with the Immunoglobulin G are shown in Figurea 3 and 4. A: acrosome (corresponding to Figure 3-B), B: a point-like organelle at the junction of the head/midpiece (corresponding to Fig. 3-D), C: midpiece (corresponding to Fig. 4-A), D: principal piece of the tail (corresponding to Fig. 4-C). Scale bars are 10 µm in length.
IIFS with partially purified IgG suggest the following perspectives: human ejaculate contains a mixture of LS and DS with substantially different features. Importantly, the antigenicity of sperm diminishes during DNA fragmentation. As DS predominates even in normozoospermic semen (Videos 1A and 1B), ASA testing of unseparated semen is subject to false negatives. According to the video of the moving cluster in Video 2, the purified LS swimming in the overcrowded suspension bumped into one another. They were less likely to form moving clusters in the unseparated semen. DS had lost their binding capacity toward all the ASAs examined (Figures 6 and 8). Denaturation of the plasma membrane [28] may cause an absence of antigenic molecules on the sperm surface and facilitate non-immunological formation of static clusters. ASA testing based on auto-agglutination requires careful interpretation, with the test specimen being rigorously limited to LS and not the unseparated semen. Among LS, the clusters that are connected to each other via the same site can then be deemed to constitute immunological auto-agglutination. Static clusters (Figure 7) that are non-specifically entangled should not be used for the diagnosis. A similar restriction should be applied to IB testing, in that the number of swimming LS attaching to the beads via the same site should be deemed ASA positive. For example, a pointlike organelle at the junction of the head/midpiece (Figure 3D) cannot be directly contacted; thus, detection of ASAs through auto-agglutination is limited to antigens on convex surfaces of the sperm. This restriction should also be applied to bulky particles using IB testing.
Video 2: Comparison of the motility and DNA integrity of live and denatured sperm, Video of Live sperm.
Note: Video 2 is recorded at 400X magnifications.
Many studies [1–15] have emphasized the clinical significance of ASAs in immune infertility, and the present study reevaluated the diagnostic criteria for ASAs. So-called “natural ASAs,” which are not etiologically significant, have also been observed in women who became spontaneously pregnant [3], and the same was observed in the present study. Figures 3 and 4 demonstrate highly antigenic organelles in human sperm, visualizing subcellular antigenic sites; however, we could not determine the etiological significance of the ASAs. Unlike monoclonal IgGs, polyclonal IgGs are often produced against an unspecified number of antigens, and the etiologically important ASAs are those that react with the molecules responsible for fertilization.
In contrast, natural ASAs react with none or slightly responsible molecules in the same organelle. Which sites interfere with the reproductive processes is still unclear, as is which molecules in such sites are responsible for deterioration of the sperm function. A comparison of the frequency of occurrence of the antigenic sites between women of infertile couples and pregnant women may reveal the etiological and non-etiological sites. IIFS of partially purified IgGs from the wife or husband reacting with the motile sperm of the husband may provide more detailed information to inspect the causes of “un-fertilization”. As the sperm obtained from the men of infertile couples often have various dysfunctions, concurrent analyses of DNA integrity and status of the acrosome and vacuoles are of service to determine whether the un-fertilization is due to dysfunctions of the sperm or immunological interference.
From a clinical viewpoint, if any of the IgGs obtained from the wife bind to any of the sites on the husband’s sperm, the therapeutic strategy should be preventively changed to in vitro fertilization/embryo transfer regardless of the etiology of ASAs, so that the sperm can merge with the oocyte without contacting the wife’s bodily fluids. If such fertilization is successful, the infertility is likely due to ASAs rather than to dysfunction of the sperm.
Use of un-separated semen for ASA testing is subject to false negatives. ASAs were detected in the sera of women of infertile couples and pregnant women. However, visualization of the subcellular locations of antigens cannot determine the etiological significance of ASAs. Which sites interfere with the reproductive processes remains unclear, as does the question of which molecules in such sites are responsible for deterioration of sperm function. Comparison of the frequency of appearance of antigenic sites between the women of infertile couples and pregnant women is the first step toward determining the etiological sites.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors declare the absence of any conflicting interests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Kaneko S, Takamatsu K (2023) Re-Evaluation of Significance of Anti-Sperm Antibodies in Clinical Immune Infertility-Antigenicity of Human Sperm Diminishes during DNA Fragmentation. J Med Diagn Meth.12:440.
Received: 31-Oct-2023, Manuscript No. JMDM-23-27851; Editor assigned: 03-Nov-2023, Pre QC No. JMDM-23-27851 (PQ); Reviewed: 17-Nov-2023, QC No. JMDM-23-27851; Revised: 24-Nov-2023, Manuscript No. JMDM-23-27851 (R); Published: 01-Dec-2023 , DOI: 10.35248/2168-9784.23.12.440
Copyright: © 2023 Kaneko S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.