Journal of Medical Diagnostic Methods
Open Access
ISSN: 2168-9784
ISSN: 2168-9784
Research Article - (2023)Volume 12, Issue 2
Objectives: To perform intercomparative studies to revalidate the principles and quantitative performance of the Sperm Chromatin Dispersion Test (SCD) and Comet Assay (CA).
Methods: Human sperm without and with granular fragments in end-stage fragmentation were purified from normozoospermic semen and used as naturally occurring negative and positive controls (NC and PC, respectively). Both SCD and CA extracted nucleoproteins with 2.0 mol/L NaCl, 1.0 mmol/L DTT. SCD determined that DNA damage as inversely proportional to the area of the violet halo. The CA estimated the level of DNA damage electrophoretically from the number of granular fragments; the so-called comet tail, their electrophoretic features were compared with the Single-Cell Pulsed-Field Gel Electrophoresis (SCPFGE).
Results: The violet halo in SCD was determined as consisting of Crystal Violet (CV)-stainable nucleoproteins that adhered to the radiated DNA fibers. SCD could not distinguish between NC and PC. The residual nucleoproteins blocked the migration of DNA in the natural CA; in contrast, SCPFGE with in-gel tryptic digestion discharged the fibrous and granular fragments beyond the elongated DNA fibers. The alkaline CA was run DNA in 0.3 mol/L NaOH. Although DNA was shredded to granular fragments, the residual nucleoproteins retained their binding capacity to DNA and still fixed the newly generated fragments.
Conclusion: DNA fragmentation analyses require measuring an early stage of fragmentation in separated motile sperm, a lack of proteolysis diminishes the quantitative performances of neutral and alkaline CA. Overall, the results suggested that SCD and CA were insufficiently sensitive as tools to harvest data for clinical statistics.
Human sperm; DNA fragmentation; Intercomparative study; Intracytoplasmic sperm injection; Single cell pulse field gel electrophoreses; Sperm chromatin dispersion test; Comet assay
PC: Positive Control; NC: Negative Control; CA: Comet Assay; SCPFGE: Single-Cell Pulsed-Field Gel Electrophoresis; CV: Crystal Violet; DTT: Dithiothreitol.
Numerous studies have discussed the impact of DNA fragmentation on sperm-derived congenital anomalies [1-3]. Male germ cells are susceptible to the accumulation of DNA lesions because apoptosis plays a crucial role in spermatogenetic quality control and the DNA repair capacity of sperm declines during late spermatogenesis [4,5]. As repairing Double-Strand Breaks (DSBs) is intrinsically more difficult than that of other DNA lesions, the critical threshold of DSBs in the nucleus may be very low [6,7]. Single-Cell Pulsed-Field Gel Electrophoresis (SCPFGE) has revealed that human semen contains a heterogeneous population of sperm with various stages of DNA fragmentation [8-10]. In the earliest stage, a few large fibrous fragments appear beyond the anterior end of a bundle of elongated long-chain fibers, and cleavage proceeds until all the DNA fibers are shredded to granular fragments. The incidence is not proportional to the number of DSBs, because those over a critical threshold cause fertilization failure or pregnancy loss. Quantitative detection of the earliest stage of DNA fragmentation in a single nucleus is a crucial diagnostic and prognostic measure in human Assisted Reproductive Technology (ART), especially in Intra Cytoplasmic Sperm Injection (ICSI) practice.
In the present study, human sperm was purified from normozoospermic semen either without DNA fragmentation or with granular fragments in the end stage of fragmentation by means of sedimentation equilibrium in Optiprep, differential velocity sedimentation in Percoll, and subsequent swim up. They were employed as the naturally occurring Negative and Positive Controls (NC and PC, respectively) as the standard preparations to validate the DNA instability analyses.
The Sperm Chromatin Dispersion (SCD) test defined that damage of DNA was inversely proportional to the area of the violet halo, and those without halos are seriously damaged [11,12]. The neutral and alkaline Comet Assays (CA) have been recognized as major tools for observing nonspecific sequential damage to DNA [13,14]. After electrophoresis, the degree of DNA damage was evaluated based on the number of granular fragments discharged from the origin, the so-called comet tail. The present study revalidated their principles and quantitative performance through intercomparative studies between NC and PC samples.
Ethics statement
Ejaculates were obtained from volunteers or patients who visited our clinic, received explanations of the aim of this study and the measurement items, and then gave consent in writing the form. The ethical committee of the Ichikawa General Hospital approved this study (approved No. 2013-03, -04).
Purification of human sperm either without DNA fragmentation or with granular fragments in the end stage of fragmentation
Sperm concentration and motility were determined according to the WHO reference manual [15]. Human normozoospermic semen (volume: 5.1 ± 0.83 mL, sperm concentration: 89 ± 13 × 106/mL, motility: 59% ± 8.0%, n=4, mean ± SD) was fractionated by means of three steps of density gradient centrifugation and subsequent the swim up. The semen specimens were diluted several times with 20 mmol/L HEPES-NaOH, Hank’s solution, pH 7.4 (hereafter called Hank’s). Optiprep (Axis-shield, Oslo, Norway) was made isotonic with 20 mmol/L Hepes-NaOH, powder type Hank’s mixture, 2.0 mg/mL Human Serum Albumin (HSA), pH 7.4, to give an apparent density of 1.17 g/ mL (hereafter called OP). The suspension was then layered on 0.5 mL of OP, centrifuged at 600 g for 10 min, and the sediment and the interface layer were re-suspended to give 1.0 mL (1.085 g/mL). It was layered on 0.5 mL of OP and ultra-centrifuged at 10,000 g for 10 min. The interface layer and sediment were recovered separately.
They diluted several times with Hank’s, then centrifuged in 90% Percoll (GE Healthcare, NJ, USA) density gradient according to our previous report [8]; 90% Percoll was made isotonic with 20 mmol/L HEPES-NaOH, pH 7.4, powder type Hanks’ mixture, 2.0 mg/mL HSA (apparent density: 1.12 g/mL, hereinafter called Percoll), then 5.0 mL Percoll was placed in a conical tip test tube and 1.0 mL of Hank’s solution was layered. It was then rotated for 10 revolutions at an angle of 30° to make a linear density gradient, the resulting sperm suspension was loaded, and it was centrifuged in a swing out rotor at 400 g for 30 min. Hank’s (1.0 mL) were layered onto the sediment fraction (0.2 mL) and allowed to stand for 60 min at room temperature. The motile sperm that swam into the upper half of the Hank’s were recovered.
Single-Cell Pulsed-Field Gel Electrophoreses (SCPFGE)
SCPFGE was performed as described in our previous reports [8- 10] with slight modifications. In brief, human sperm that adhered to agarose-coated glass slides were embedded in molten 0.5% agarose containing 20 μg/mL highly purified bovine pancreatic trypsin (50 mmol/L acetate buffer, pH 4.7) to form a 100-μmthick gel coating. After being chilled, the gel film was incubated in the cell lytic reagent (50 mmol/L Tris-HCl, 8.2 mmol/L hexa-metaphosphate Na, 0.05% Triton X-100, 5.0 mmol/L dithiothreitol, pH 8.2) at 37°C for 30 min. The apparatus was equipped with dual electrode pairs arranged at a 60° angle, and the gel film on the glass slide was positioned at the cross-point of the electric currents. The currents were applied at 2.0 V/cm with 8.0-s switching intervals for 7 min in electrophoretic buffer (30 mM Tris-acetate, pH 8.2). The DNA in the gel was stained with diluted (1 × 104) SYBR gold (Molecular Probes, Eugene, OR, USA) and observed under an epifluorescence microscope with a green filter (Axio Imager A1, Carl Zeiss MicroImaging, Jena, Germany). Still images were recorded using a high-resolution charge-coupled device camera (AxioCam HRC, Carl Zeiss MicroImaging). Based on their size, the microscopic features of DNA were classified as long-chain fibers and fibrous and granular fragments, as described previously [8]. Sperm with at least one fibrous fragment under the microscope were classified as damaged, and more than 200 sperm were counted to determine the DNA damage rate.
The Sperm Chromatin Dispersion (SCD) test and the Comet Assay (CA)
The sample preparation for SCD and CA was performed in the same manner; the sperm adhered to the agarose coated glass slide were embedded in molten 1.0% agarose, 50 mM Tris-acetate, pH 8.2, to form a 100 μm-thick gel coating [11-14]. After being chilled, the gel film was immersed in the lytic medium (50 mmol/L Tris- HCl, 2.0 mol/L NaCl, 1.0 mmol/L DTT, 0.05% Triton-X 100, pH 8.2) at 37°C for 20 min.
SCD: The lysed sperm was stained with 0.1% Crystal Violet (CV), 50 mmol/L Tris-HCl, pH 8.2, for 20 min. After washing out the excess dye, images were recorded under a bright-field microscope. SCD indicates that the damage to DNA is inversely proportional to the area of the halo, and those without a halo are seriously damaged.
The neutral and alkaline CA: After washing out the lytic medium, the neutral CA and the alkaline CA run the gel under monodirectional current-carrying at 1.3 V/cm for 3 min in 30 mM Tris-acetate, pH 8.2, or 0.3 mol/L NaOH, respectively. The gels were then stained, and fluorescent images were recorded in the same manner as SCPFGE.
Acid polyacrylamide gel electrophoresis of protamine
The protamine sulphate salt from herring (10 µg/well, Sigma- Aldrich, St. Louis, MO, USA) was electrophoresed in 18% polyacrylamide gel, pH 3.7, with 0.1% CV as the leading marker [16]. After the separation, the gel was stained with 0.02% Coomassie Brilliant Blue G-250 according to a common procedure or with 0.1% CV, 50 mmol/L Tris-HCl, pH 8.2, for 120 min.
Figure 1 shows a summary of the centrifugal separation process. The diluted semen was condensed (Figure 1A), then was fractionated by means of the sedimentation equilibrium in OP (Figure 1B) and differential velocity sedimentation in Percoll (Figures 1C and 1D). The sperm from the sediment of OP/ intermediate layer of Percoll were almost immotile and auto agglutinated (Figure 1D), and the apparent density was estimated to exceed 1.17 g/mL (Figure 1B). The sperm recovered from the interface layer of OP/sediment of Percoll was progressively motile with a mean of 83% ± 2.7%, and the apparent density was estimated to be in the range of 1.12-1.17 g/mL (Figures 1B and 1C). The final preparation with full motility (95% ± 2.1%) was prepared from the sediment of Percoll using the swimup method. SCPFGE revealed that the unseparated semen contained a heterogeneous sperm population with various stages of DNA fragmentation (Figure 2A); 85 ± 1.9% of the sperm in the final preparation were elongated with a bundle of long chain fibers from the origin without any fragments (Figure 2B); the remainder showed a few to several dozen fibrous fragments beyond the anterior end of the elongated fibers. Figure 2C shows an enlarged image; DNA fibers followed serpentine tracks by pulsed-field impression. In contrast, all sperm in the sediment of the OP/intermediate layer of Percoll were discharged granular fragments from the origin (Figure 2D). The final preparation recorded the highest negative rate of DNA fragmentation (88%; 1.0 mL, 18 × 106 sperm/mL, 98% motility) was employed as NC and the same specimen provided PC (less than 3%; 1.0 mL, 48 × 106 sperm/mL, less than 5% motility).
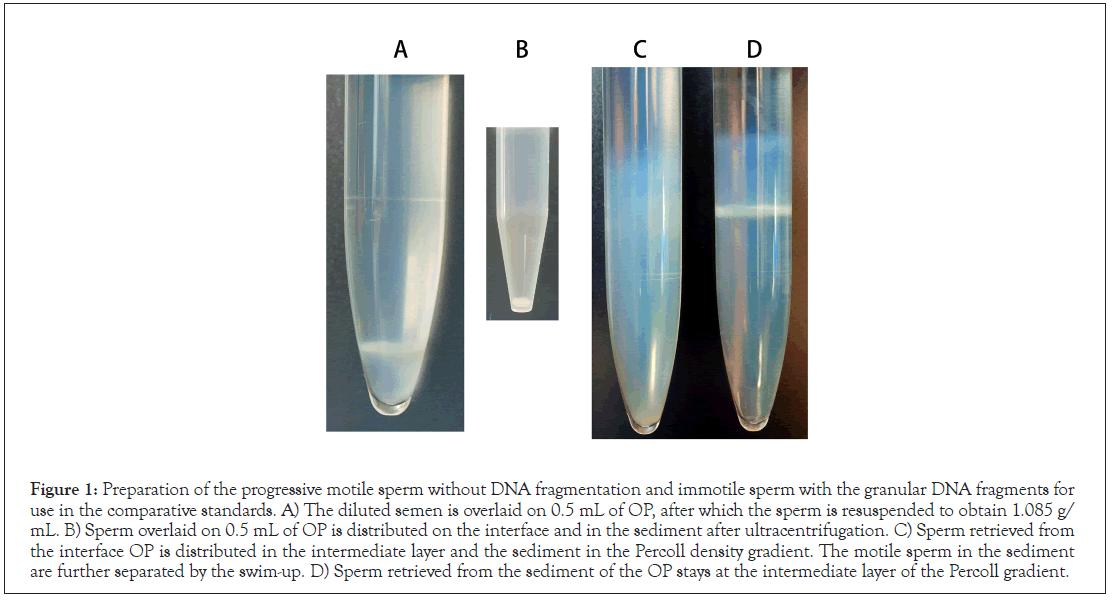
Figure 1: Preparation of the progressive motile sperm without DNA fragmentation and immotile sperm with the granular DNA fragments for use in the comparative standards. A) The diluted semen is overlaid on 0.5 mL of OP, after which the sperm is resuspended to obtain 1.085 g/ mL. B) Sperm overlaid on 0.5 mL of OP is distributed on the interface and in the sediment after ultracentrifugation. C) Sperm retrieved from the interface OP is distributed in the intermediate layer and the sediment in the Percoll density gradient. The motile sperm in the sediment are further separated by the swim-up. D) Sperm retrieved from the sediment of the OP stays at the intermediate layer of the Percoll gradient.
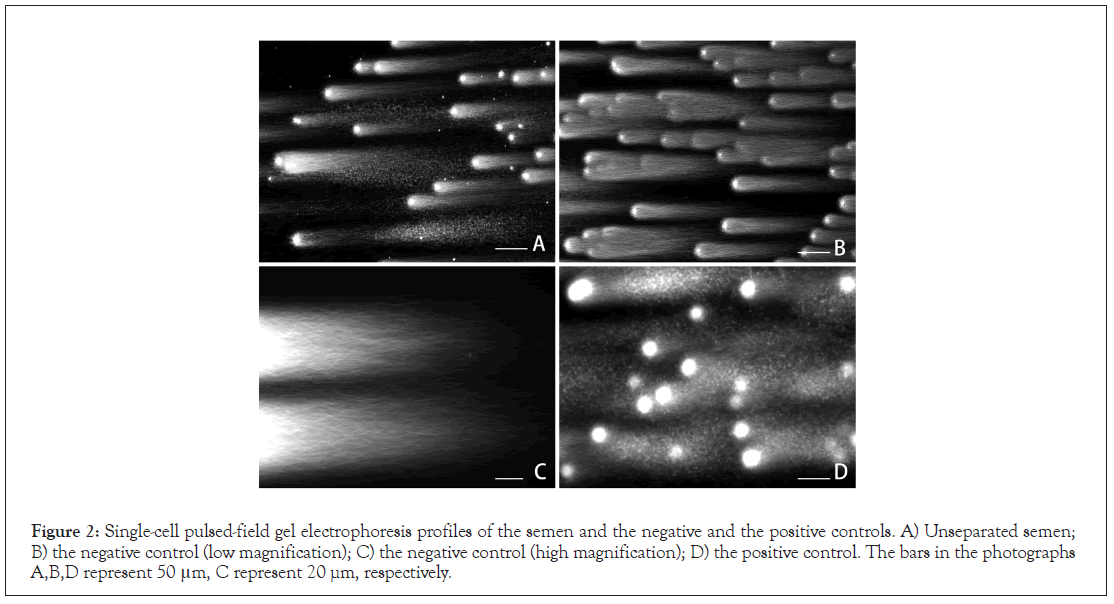
Figure 2: Single-cell pulsed-field gel electrophoresis profiles of the semen and the negative and the positive controls. A) Unseparated semen; B) the negative control (low magnification); C) the negative control (high magnification); D) the positive control. The bars in the photographs A,B,D represent 50 μm, C represent 20 µm, respectively.
The principles and quantitative performance of SCD and CA were revalidated using intercomparative studies. SCD denotes that sperm without a halo are seriously damaged. As shown in Figure 3A, the sperm without halo was found in the unseparated semen and recovered in PC, but not in NC (Figures 3B and 3C). NC was stained deeply with CV and was radiated out of the uniformly sized halo (Figure 3B); in contrast, PC showed a halo of various sizes (Figure 3C). In the present study, we could not purify sperm without a halo, the features of which remain unclear. NC stained with CV was washed with 80% ethanol, the color of the head faded, and the halo disappeared (Figure 3D). Hydrophobic forces might contribute to the interaction between CV and sperm components. The features of DNA in NC and PC suggested that they corresponded to the initial and end stages of DNA fragmentation (Figure 2), whereas the changes in the sizes of the halo were not particularly obvious between the comparative standards (Figure 3).
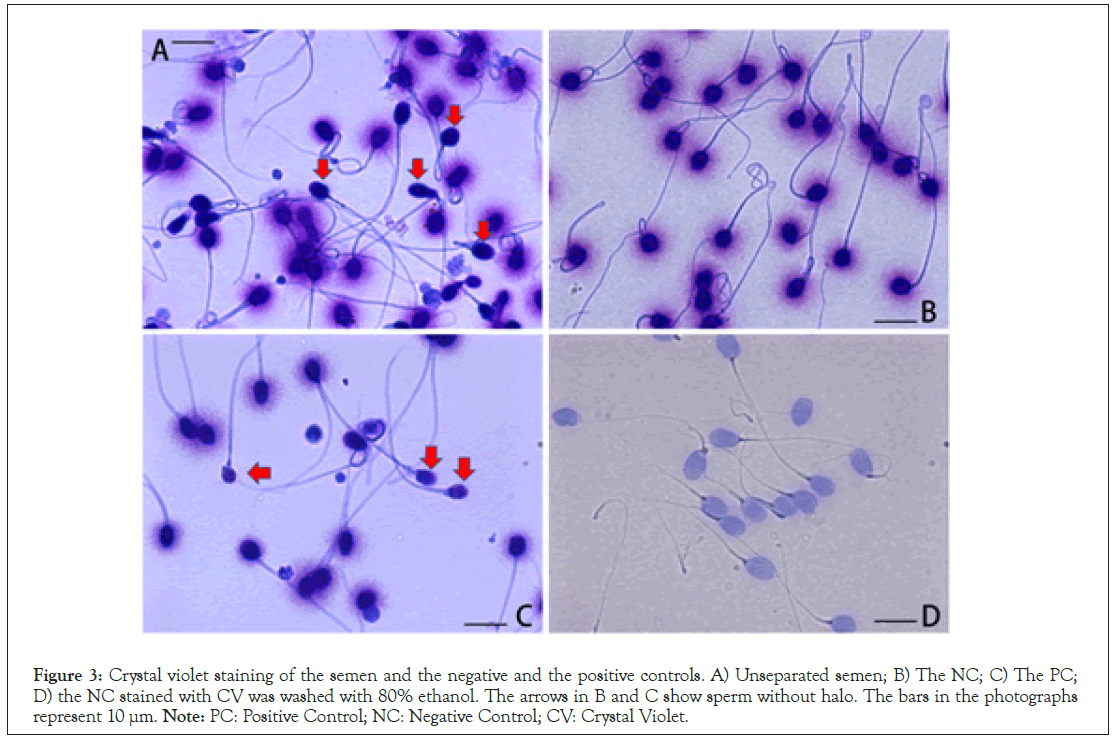
Figure 3: Crystal violet staining of the semen and the negative and the positive controls. A) Unseparated semen; B) The NC; C) The PC; D) the NC stained with CV was washed with 80% ethanol. The arrows in B and C show sperm without halo. The bars in the photographs represent 10 µm. Note: PC: Positive Control; NC: Negative Control; CV: Crystal Violet.
Nucleoproteins in human sperm consist of protamine, histone, condesin, and cohesin [17-19]. The lytic medium used in SCD protocol is a popular formula for extracting protamine [11- 14,16,17]. The embedded NC was incubated in the modified lytic medium that replaced 2.0 mol/L NaCl, a monovalent ion pair, to 8.2 mmol/L hexa-metaphosphate Na, a poly-multivalent anion. As shown in Figure 4A, the head was swollen, and the halo disappeared. NC was embedded in molten 1.0% agarose containing the lytic medium, and then it was kept at 37°C for 20 min to facilitate free diffusion of DNA fibers. After chilling, the gel was stained with CV. As shown in Figure 4B, some components that were unextractable with 2.0 mol/L NaCl and stainable with CV still adhered to the elongated DNA fibers. Since trypsin was inhibited in the presence of 2.0 M NaCl, in-gel tryptic digestion was performed in the presence of 8.2 mmol/L hexa-metaphosphate Na according to SCPFGE protocol. The CV-stainable components in the head were completely degraded, whereas the tail was tolerant of tryptic digestion (Figure 4C).
Protamine, a strongly basic protein, was separated in acidic polyacrylamide gel and stained with 0.02% Coomassie Brilliant Blue G-250, an acidic dye (Figure 4Da). The electrophoretic motilities of CV (Figure 4Db) and protamine were similar. On one hand, 0.1% CV, a basic dye, did not stain protamine at pH 8.2 (Figure 4Dc). The above results suggest that at least a part of the other three nucleoproteins (histone, condesin, cohesion) stained with CV might visualize the radiated DNA fibers as a violet halo (Figure 3).
The electrophoretic profiles of NC and PC were compared between the neutral CA and SCPFGE. The typical comet tail shown in the literature [13,14] was obtained by running at 1.3 V/cm for 3 min under mono-directional current-carrying (Figure 5A). Both methods discharged granular fragments from PC (Figures 2D and 5A), although the amount was beyond the comparison. In contrast to SCPFGE (Figures 2B and 2C), the neutral CA could not discharge DNA from NC (Figure 5B). SCD and CA employed a similar protocol to extract the nucleoproteins, and the results in Figure 4 suggest that those unextractable with 2.0 mol/L NaCl were still adhered to DNA fibers (Figure 4C). NC and PC were embedded in 1.0% agarose, and then were ingel digested according to SCPFGE protocol. The naked DNA fibers in NC were run at 1.3 V/cm for 3 min or 2.0 V/cm for 7 min under mono-directional current-carrying; the former gave a profile similar to that shown in Figure 5B (data not shown); the latter pulled out the short fibers from the origin (Figure 5C). When compared with Figures 5A and 5D, tryptic digestion induced the significant increase of granular fragments from PC at the run with 1.3 V/cm for 3 min under mono-directional currentcarrying. The results in Figure 5 suggest that the nucleoproteins fixed the DNA fibers and fibrous fragments, blocking their electrophoretic migration completely. Even at the end stage of fragmentation, the majority of granular fragments were still fixed with the nucleoproteins, and a small part of them escaped from the fixation were allowed to discharge from the origin.
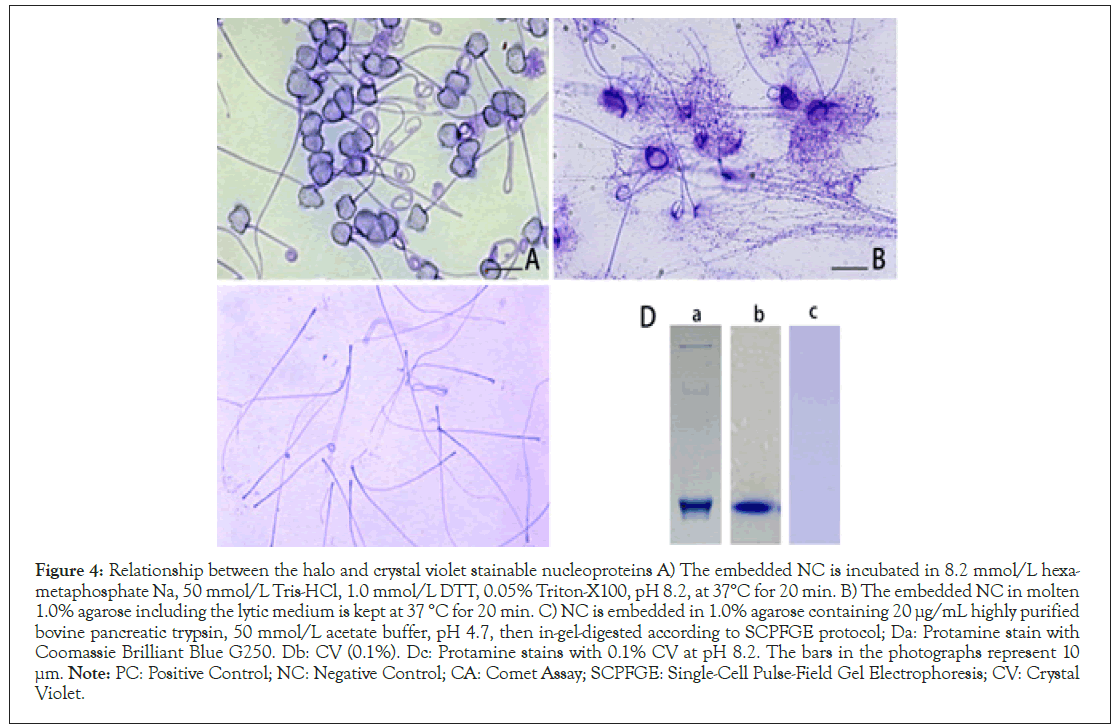
Figure 4: Relationship between the halo and crystal violet stainable nucleoproteins A) The embedded NC is incubated in 8.2 mmol/L hexametaphosphate
Na, 50 mmol/L Tris-HCl, 1.0 mmol/L DTT, 0.05% Triton-X100, pH 8.2, at 37°C for 20 min. B) The embedded NC in molten
1.0% agarose including the lytic medium is kept at 37 °C for 20 min. C) NC is embedded in 1.0% agarose containing 20 μg/mL highly purified
bovine pancreatic trypsin, 50 mmol/L acetate buffer, pH 4.7, then in-gel-digested according to SCPFGE protocol; Da: Protamine stain with
Coomassie Brilliant Blue G250. Db: CV (0.1%). Dc: Protamine stains with 0.1% CV at pH 8.2. The bars in the photographs represent 10 μm. Note: PC: Positive Control; NC: Negative Control; CA: Comet Assay; SCPFGE: Single-Cell Pulse-Field Gel Electrophoresis; CV: Crystal
Violet.
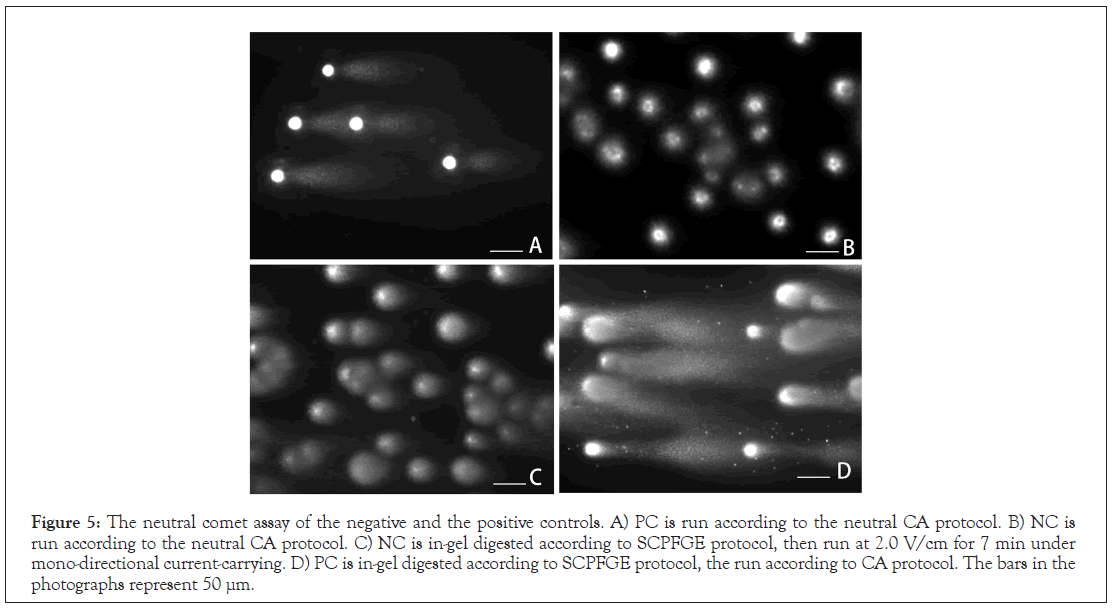
Figure 5: The neutral comet assay of the negative and the positive controls. A) PC is run according to the neutral CA protocol. B) NC is run according to the neutral CA protocol. C) NC is in-gel digested according to SCPFGE protocol, then run at 2.0 V/cm for 7 min under mono-directional current-carrying. D) PC is in-gel digested according to SCPFGE protocol, the run according to CA protocol. The bars in the photographs represent 50 μm.
To observe the effect of alkaline treatment on DNA fibers, the embedded NC run under the alkaline CA protocol, as shown in Figure 6A, there found no noticeable change from the profile shown in Figure 5B. When the in-gel digested NC was incubated in 0.3 mol/L NaOH for 10 min, then run according to the CA protocol, the naked DNA fibers were shredded to granular fragments (Figure 6B). The binding capacities of the nucleoproteins to DNA were tolerant to the radical treatment with 0.3 mol/L NaOH, and they blocked the migration of the neogenerated granular fragments. The head of the sperm maintained stainability for CV (Figure 6C). To examine the tolerance of the phosphodiester bond to alkaline hydrolysis, the in-gel digested NC was treated with 10 mmol/L NaOH for 10 min at room temperature. After neutralization, the gel was run accroding to SCPFGE protocol. As shown in Figure 6D, short fibrous and granular fragments were observed beyond the elongated DNA fibers.
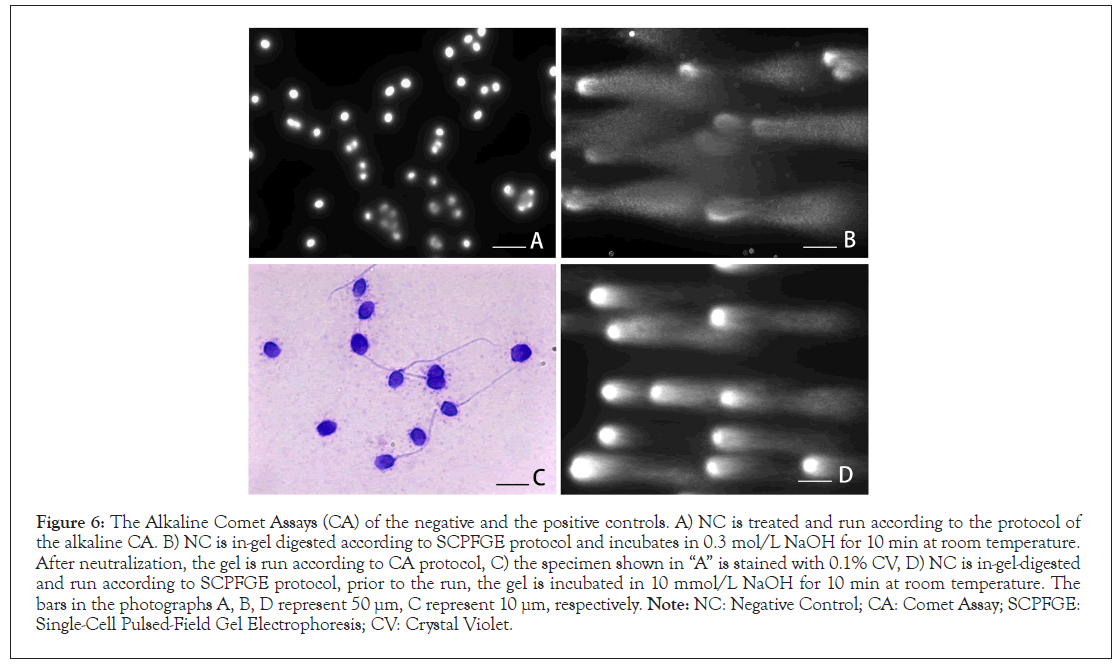
Figure 6: The Alkaline Comet Assays (CA) of the negative and the positive controls. A) NC is treated and run according to the protocol of the alkaline CA. B) NC is in-gel digested according to SCPFGE protocol and incubates in 0.3 mol/L NaOH for 10 min at room temperature. After neutralization, the gel is run according to CA protocol, C) the specimen shown in “A” is stained with 0.1% CV, D) NC is in-gel-digested and run according to SCPFGE protocol, prior to the run, the gel is incubated in 10 mmol/L NaOH for 10 min at room temperature. The bars in the photographs A, B, D represent 50 μm, C represent 10 μm, respectively. Note: NC: Negative Control; CA: Comet Assay; SCPFGE: Single-Cell Pulsed-Field Gel Electrophoresis; CV: Crystal Violet.
To date, many cohort follow-up studies have nominated DNA fragmentation in human sperm nuclei as a major risk factor for sperm-derived congenital anomalies [1-3]; SCD and CA have been widely used to harvest statistical results in cohort studies [11-14]. The present study arose from the simple question that granular fragments in the comet tail were derived from the immotile sperm in the end stage of fragmentation. Prior to drawing a conclusion to this issue, we revalidated the measurement principles and quantitative performances of SCD and CA by means of intercomparative studies. Apoptosis plays a crucial role in spermatogenetic quality control [4]; and the following symptoms indicate that NC and PC might contain the sperm that have not yet and have already undergone apoptosis, respectively. The release of cytochrome c from mitochondria, an initiative step in apoptosis, immobilizes sperm [20]. Cell shrinkage by apoptotic volume decrease increases the apparent density [21]. PC with granular fragments was almost immotile and was heavier than 1.17 g/mL. The externalization of phosphatidylserine is also recognized as an apoptotic marker, and PC is auto-agglutinated (Figure 1D). NC without fragments or with a small number of fibrous fragments was almost motile, lighter than PC, and did not auto-agglutinate.
The results showed Figures 3 and 4 suggest that the violet halo was identified as the radiated DNA fibers visualized by the adhered nucleoproteins, except for protamine. Not only DNA fibers in NC, but also the majority of the granular fragments in PC, were still fixed with nucleoproteins (Figures 5A-5D), and only a small portion of the fragments that escaped fixation were discharged (Figures 5A-5D). The halo in PC (Figure 3C) suggested that some of the DNA existed as fibrous fragments, even in the end stage of fragmentation. The change in the sizes of the halo was not obvious before and after fragmentation (Figures 3B and 3C), and SCD could not indisputably distinguish NC and PC. The direct observation of DNA by the aid of the electrophoretic methods may be more specific and sensitive compared to indirect measurement through the adhered nucleoproteins on DNA.
Neutral CA and SCPFGE electrophoretically analyze DNA fragmentation in a single nucleus, and the assessment criteria differ from each other; the former estimates the level of DNA damage from number of granular fragments, while the latter simultaneously observes fibrous and granular fragments separated beyond the elongated DNA fibers. The overall results shown in Figures 5 and 6 suggest that a lack of tryptic digestion made a crucial difference between CA and SCPFGE. Since CV did not stain the protamine (Figure 4D); at least a part of histone, condesin, and cohesion blocked the electrophoretic migration of DNA in the neutral CA.
The residual nucleoproteins also caused confusion in the alkaline CA. The phosphodiester bonds were fragile to alkaline hydrolysis, and 10 mM NaOH cleaved the DNA fibers into fibrous and granular fragments (Figure 6D). The amount of neo-generated fragments after radical treatment with 0.3 mol/L NaOH (Figure 6B) was comparable to that in PC (Figure 5D), and the nucleoproteins still fixed the neo-generated fragments (Figure 6A). CV was destained with ethanol (Figure 3D) but was tolerant to high alkalinity (Figure 6C); meanwhile, hydrophobic interactions might contribute to the binding capacity of CV to besides ionic forces.
Alkaline-based Single-Strand Break (SSB) assays lead to the cleavage of DNA strands at Alkaline-Labile Sites (ALS), including the Apurinic Site (APS) [22,23]. The motile sperm fraction corresponding to NC is routinely provided for clinical in vitro fertilization and ICSI, and there are few possibilities that ALS or APS occupy a major part of the DNA sequence. DNA fluorescent dyes were not absolutely distinguishable from single and double strands. A major part of the fragments might have been neogenerated by the alkaline hydrolysis of the double strand, but they were still fixed with the nucleoproteins due to a lack of tryptic digestion. The alkaline CA is associated with the risk of SSB overestimation. ALS or APS should be identified with specific APS detection methods, TUNEL assays, and anti-single strand DNA monoclonal antibodies [24-26].
The above discussion emphasizes the significance of proteolytic digestion, and we noted other key points to ensure the quantitative performance of DNA fragmentation analyses. First, pulsed-field electrophoresis in lower concentration agarose (0.5%) at a higher voltage (2.0 V/cm) was essential for observing DNA fibers and the fibrous and the granular fragments simultaneously. Second, the cytological competence of test objects should be rigorously defined. DNA fragmentation over the critical threshold causes pregnancy failure; thus, it is necessary to detect the early stage of fragmentation in the separated motile sperm to ascertain clinical statistics in cohort studies. The comet tail (Figure 5A) might be derived from immotile sperm at the end stage of fragmentation; they do not contribute to pregnancy. Once embedded and lysed, it is difficult to identify the types of sperm from which the DNA are derived. Unseparated semen is disqualified as a test sample.
In conclusion, intercomparative studies suggested that the size of the halo in the SCD was not obviously different between NC and PC, and the lack of proteolysis diminished the quantitative performance of the neutral and the alkaline CA. Therefore, both SCD and CA were disqualified as analytical tools to discuss sperm-derived congenital anomalies in ART.
Author has nothing to disclose.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Kaneko S, Takamatsu K (2023) Revalidation of the Sperm Chromatin Dispersion Test and the Comet Assay Using Intercomparative Studies Between Purified Human Sperm without and with End-Stage DNA Fragmentation. J Med Diagn Meth. 12:406.
Received: 13-Mar-2023, Manuscript No. JMDM-23-22233; Editor assigned: 16-Mar-2023, Pre QC No. JMDM-23-22233 (PQ); Reviewed: 03-Apr-2023, QC No. JMDM-23-22233; Revised: 13-Apr-2023, Manuscript No. JMDM-23-22233 (R); Published: 24-Apr-2023 , DOI: 10.35248/2168- 9784.23.12.406
Copyright: © 2023 Kaneko S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.