Journal of Medical Diagnostic Methods
Open Access
ISSN: 2168-9784
ISSN: 2168-9784
Research Article - (2023)Volume 12, Issue 1
Objectives: The WHO reference manual for andrology considers the outline of sperm head as a key parameter for assessing male infertility and classifies internal vacuoles as head defects. The features of vacuoles were heterogeneous among interindividual as well as inter-sperm. The present study reported some unique properties of Reactive Blue 2 (RB2). The dye exhibited pH-dependent cellular specificity for human sperm and spermatid.
Materials and Methods: Human sperm and spermatid were stained with RB2 at pH 10.0.
Results: RB2 stained human sperm and spermatids, which appeared as translucent bluish bodies, but did not stain lymphocytes, while inner vacuoles appeared as toneless spots. RB2 staining also revealed degraded spermatids that had undergone protamination but were arrested prior to tail elongation. The majority of azoospermic semen specimens (16/23 cases) included sperm or degraded spermatids. Although the features of the head outline and vacuoles were heterogenous among interindividual, RB2 staining in the presence of 2.0 mmol/L Dithiothreitol universally re-formed the outline into an oval shape and led to the disappearance of the vacuoles, regardless of their original features.
Conclusion: Three sulfate residues in RB2 interacted selectively with guanidyl residues in Arg of protamines at pH 10. The cellular specificity was due to the Arg content of the nucleoproteins. RB2 revealed the head outline and internal vacuoles of the sperm and arrested spermatids those had undergone protamination. Local failure of disulfide cross-linkage might play a critical role in vacuole formation. RB2 staining opened new methods for exploring spermiogenesis.
Reactive Blue 2; Protamine; Human sperm; Sperm vacuole; Protamine; Azoospermia; Teratozoospermia; Disulfide Bond Cross Linkage
The term “semen quality” has been used as a surrogate parameter for male reproductive function. The WHO reference manual for andrology considers the morphology of the sperm head as a key parameter for assessing male infertility; an oval head is defined as normal according to the strict criteria given in Kruger et al. [1,2]. The manual also classifies internal vacuoles as head defects.
With Reactive Blue 2 (RB2) staining, human sperm heads appeared as translucent bluish bodies, with their inner vacuoles as toneless spots, under normal bright field optics at neutral pH [3]. Further, fluorescent DNA staining indicated that the spots represented low-density areas of DNA [3].
During the late haploid phase of spermatogenesis when mammalian sperm undergoes protamination, nucleoproteins are replaced from histones to protamines [4]; arginine (Arg)-rich domains in nucleoproteins bind to the phosphoric group in DNA [5]. RB2 stained human sperm, but not lymphocytes, at pH 10. Three sulfate residues in the RB2 molecule bound electrostatically with guanidyl residues in Arg, but not with amino residues in Lys at this pH. The pH-dependent cellular specificity was due to differences in Arg content between protamines and histones.
RB2 revealed that the features of vacuoles were heterogeneous among interindividual as well as inter-sperm. Degraded spermatids that had undergone protamination but were arrested prior to tail elongation appeared as bluish bodies after RB2 staining. Intra- and inter-cross-linkage of cysteine in protamines promotes sperm head condensation and DNA stabilization [5]. RB2 staining revealed that disulfide bond reduction with Dithiothreitol (DTT) uniformly reformed the head outline into an oval shape and led to the disappearance of the vacuoles regardless of their original features. This fact suggested that local failure of the disulfide cross-linkage might be involved in vacuole formation. The novel properties of RB2 opened new approaches for exploring male infertility.
Staining of human sperm and lymphocytes
Ejaculates were obtained from patients who visited the Reproduction Center, Ichikawa General Hospital, Tokyo Dental College. The Ethical Committee of Ichikawa General Hospital specifically approved this study (approval number: I 19-68). Sperm concentration and motility were measured according to methods specified in the WHO reference manual. Seminal plasma was excluded prior to staining. A 20% Percoll (GE Healthcare, NJ, USA) solution was made isotonic with 20 mM HEPES-buffered Hank’s solution at pH 7.4. The semen was diluted 4–5 times with saline, layered on 3 mL of isotonic 20% Percoll, and centrifuged in a swing-out rotor at 400 ×g for 10 min. The sediment was designated as unseparated cells. Azoospermic semen was also processed in the same manner.
Human sperm with progressive motility was prepared as described in previous reports [6]. Diluted semen was layered on 90% isotonic Percoll density gradient and centrifuged in a swing- out rotor. The sperm recovered in the sediment was subsequently separated using the swim-up method.
Human peripheral blood (2.0 mL) was diluted twice with saline containing 1.0 mmol/L EDTA, layered on 4.0 mL of Lymphoprep (Nycomed Pharma, Zurich, Switzerland), and centrifuged at 600 ×g for 10 min. The lymphocytes at the intermediate layer were obtained.
The unseparated cells, the lymphocytes, and the purified motile sperm were diluted with saline, adhered on a plain glass slide using a centrifugal auto-smear (Cyto-Tek; Sakura, Tokyo, Japan), and fixed with methanol for 5 min. The specimens were stained with 0.02% RB2 (0.1 mol/L Na2CO3-NaHCO3, pH 10.0) for 10 min, followed by washing out of the excess dye with pure water.
The images were observed under oil immersion using a bright- field transmitted light upright microscope (Axio Imager A1; Carl Zeiss Micro-imaging, Jena, Germany; ×1000 magnification). Still images were recorded using a high-resolution charge-coupled device camera (AxioCam HRC; Carl Zeiss Micro-imaging, Germany).
Staining of mature mouse sperm in the cauda epididymis and spermatids in the testes
Fifteen-week-old male ICR mice (CLEA Japan, Tokyo, Japan) were sacrificed by dislocation and then their testes and cauda epididymis were isolated. Using small scissors, the cauda epididymis was minced in 10 mM HEPES-buffered saline. The motile sperm dispersed in the medium were adhered on the glass slide in the same manner as the human sperm. The testes were fixed in modified Davidson’s fluid within 24 hour [7]. Subsequently, they were processed with graded alcohol, cleared in xylene, and embedded in paraffin. Sections (4 μm thick) were prepared and adhered on the glass slide.
The mouse sperm and testes sections were stained with 0.02% RB2 (0.1 mol/L Na2CO3-NaHCO3, pH 10.0) for 30 min, followed by washing out of the excess dye with pure water. The images were observed in the same manner as the human specimens.
At pH 10, RB2 stained human sperm heads blue and the inner vacuoles as toneless spots, whereas the lymphocytes and other cells and particles in human semen remained scarcely stained (Figure 1). This specificity was lost at neutral pH (50 mmol/L HEPES-NaOH, pH 7.4), and the lymphocytes and all cells in the semen were also stained blue.
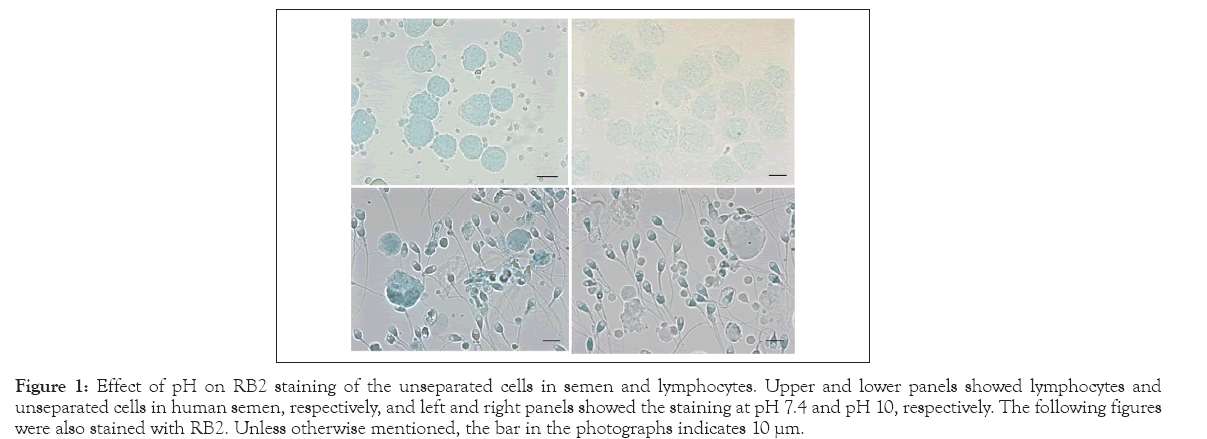
Figure 1: Effect of pH on RB2 staining of the unseparated cells in semen and lymphocytes. Upper and lower panels showed lymphocytes and unseparated cells in human semen, respectively, and left and right panels showed the staining at pH 7.4 and pH 10, respectively. The following figures were also stained with RB2. Unless otherwise mentioned, the bar in the photographs indicates 10 μm.
To ascertain the reason for pH dependency in cellular specificity, we focused on the interaction between the three sulfate residues in RB2 and Arg in protamine. RB2 (0.1%, 0.1 mol/L Na2CO3-NaHCO3 , pH 10.0) was mixed with equal volumes of protamine from herring (1.0 mg/mL) or crude histones from the calf thymus (1.0 mg/mL). Protamine formed a gluey cluster, whereas histone formed no aggregates.
Figure 2 shows the sperm with a normal outline (Figure 2A) and with various morphological aberrations (Figures 2B-2D). Even in the un-separated semen, the majority of sperm in Figure 1A shows a smooth oval-shaped head, with or without a pinpoint vacuole. As shown in Figures 2B and 2C, the disorder of spermatogenesis deteriorated not only the outline of the head, but also the number and shape of the inner vacuoles. Figure 2D presents the sperm of a patient diagnosed with severe oligozoospermia (40 × 104 sperm/ mL); small round cells stained bluish were rather conspicuous compared with the sperm with tail. The present method revealed degraded spermatids that had undergone protamination but were arrested prior to tail elongation.
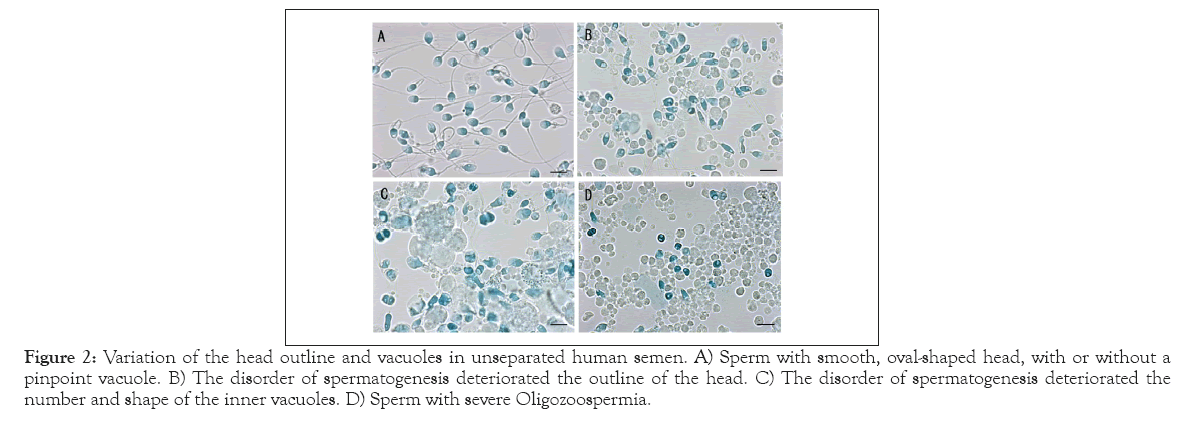
Figure 2: Variation of the head outline and vacuoles in unseparated human semen. A) Sperm with smooth, oval-shaped head, with or without a pinpoint vacuole. B) The disorder of spermatogenesis deteriorated the outline of the head. C) The disorder of spermatogenesis deteriorated the number and shape of the inner vacuoles. D) Sperm with severe Oligozoospermia.
Percoll density gradient centrifugation with subsequent swim-up is a popular method for separating motile sperm using assisted reproductive technology. We collected four specimens with motility of >90% following the swim-up (Figure 3). Immotile sperm and non-sperm debris were excluded from the specimens. The majority of sperm in Figures 3A-3B had an oval-shaped head, whereas the features of vacuoles were different from each other. A small part of the sperm in Figure 3A presents a pinpoint vacuole, whereas more than half of the sperm in Figure 3B present large vacuoles in the acrosomal region. Figure 3C exhibits sperm with a slender-shaped head and vacuoles of various sizes, and small- sized vacuoles were sporadically found in Figure 4D. Figures 2 and 3 suggested that the features of vacuoles were heterogeneous among inter-individual as well as inter-sperm, and the swim-up method could not exclude the motile sperm with vacuoles.
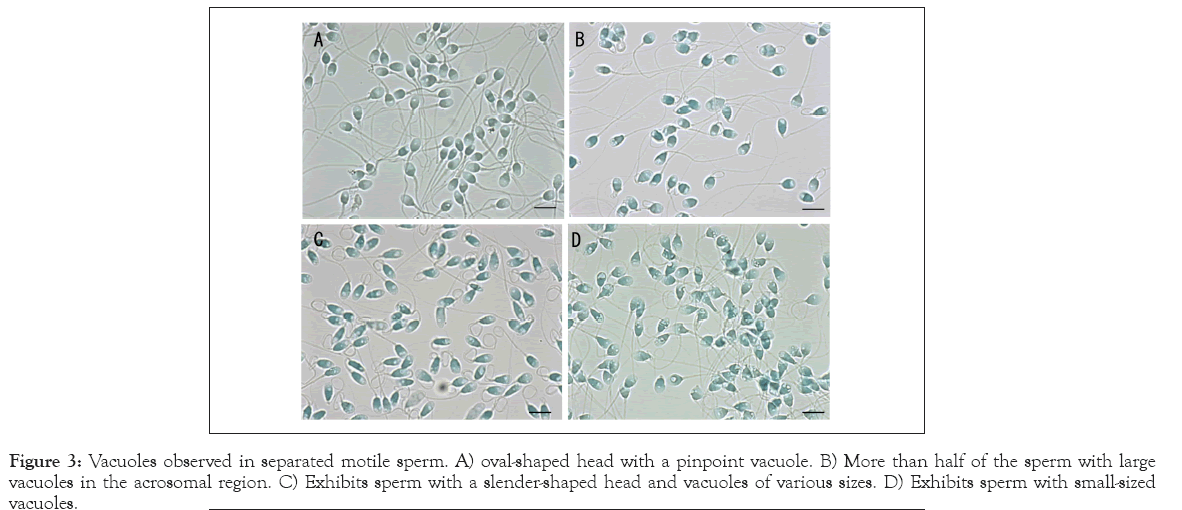
Figure 3: Vacuoles observed in separated motile sperm. A) oval-shaped head with a pinpoint vacuole. B) More than half of the sperm with large vacuoles in the acrosomal region. C) Exhibits sperm with a slender-shaped head and vacuoles of various sizes. D) Exhibits sperm with small-sized vacuoles.
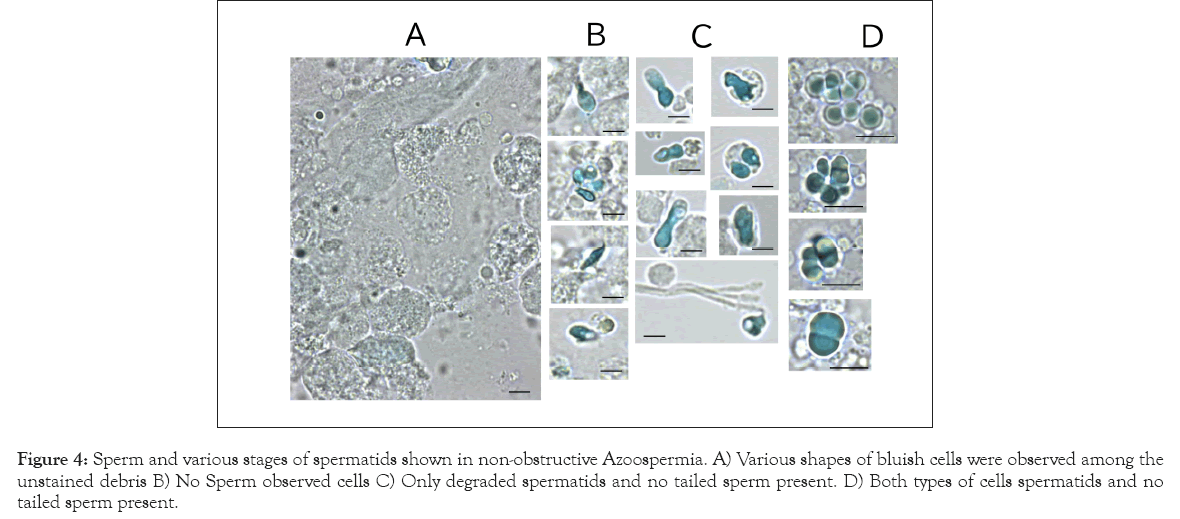
Figure 4: Sperm and various stages of spermatids shown in non-obstructive Azoospermia. A) Various shapes of bluish cells were observed among the unstained debris B) No Sperm observed cells C) Only degraded spermatids and no tailed sperm present. D) Both types of cells spermatids and no tailed sperm present.
The WHO reference manual recommends centrifugal concentration prior to observing oligozoospermia and azoospermia [8]. The precipitates were transparent under phase-contrast optics, and debris overlap interfered with sperm observation. Moreover, technicians cannot recognize spermatids without the tail. As shown in Figure 2D, RB2 staining revealed degraded spermatids that had undergone protamination but were arrested prior to tail elongation. We reinvestigated 23 semen specimens previously diagnosed as non-obstructive azoospermia using conventional methods. More than 30 field-of-view observations were used to inspect the bluish cells. The term non-obstructive azoospermia often indicates that no sperm is present in the ejaculate because of the abolition of spermatogenesis. Various shapes of bluish cells were observed among the unstained debris (Figure 4A). Figures 4B-4D summarizes three typical cases. In particular, the cells in Figures 4C and 4D could not be recognized as spermatids without RB2 staining. The specimens were classified into three groups: no observed cells (7 cases); only degraded spermatids, and no tailed sperm present (9 cases); and both types of cells present (7 cases). The quantity and features of the bluish cells differed substantially among specimens. The novel RB2 staining technique at pH 10 suggested that more than half of the patients who were diagnosed with azoospermia by the conventional method involved some cells with protamines.
Inter-disulfide and intra-disulfide crosslinking of protamine is essential for the condensation and organization of the shape of the mammalian sperm head [9]. Figure 5 presents the effects of the disulfide bond reducing agent on the head outline and vacuoles. The upper panels present the un-separated sperm with an abnormal outline and vacuoles, and the lower panels present the separated motile sperm with an oval-shaped head. RB2 staining in the presence of 2 mmol/L DTT led to the swelling and re-formation of the head into an oval shape and the disappearance of the vacuoles. These phenomena were observed universally in all sperm regardless of their original features.
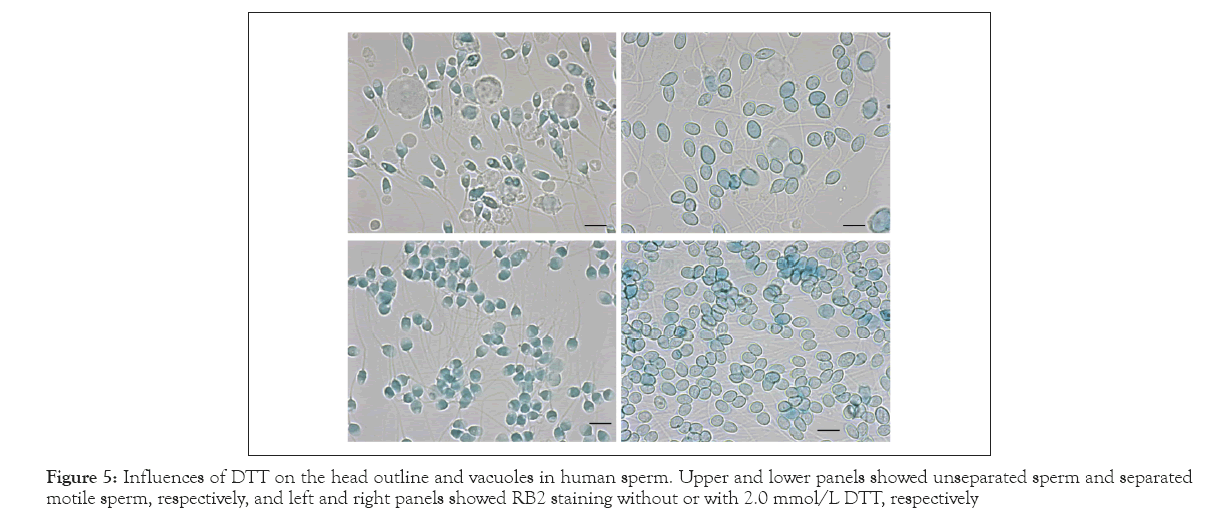
Figure 5: Influences of DTT on the head outline and vacuoles in human sperm. Upper and lower panels showed unseparated sperm and separated motile sperm, respectively, and left and right panels showed RB2 staining without or with 2.0 mmol/L DTT, respectively
Figure 6 presents the staining properties of RB2 toward the mouse sperm and spermatids in the seminiferous tubule. With RB2, the mouse sperm head was also stained blue (Figure 6A), although the tone was lighter than that of the human sperm head. No vacuole was observed in almost all the sperm retrieved from the cauda epididymis. RB2 selectively stained spermatids near the inner cavity of the seminiferous tubule (Figure 6B). In contrast to the human sperm head outline (Figure 5), 2 mmol/L DTT did not transform the mouse sperm head outline (Figure 6C). Further addition of 200 mmol/L Na2SO4, a dissociating agent of the DNA–protamine complex, facilitated swelling of mouse sperm, but the characteristic shape of the sperm remained the same.
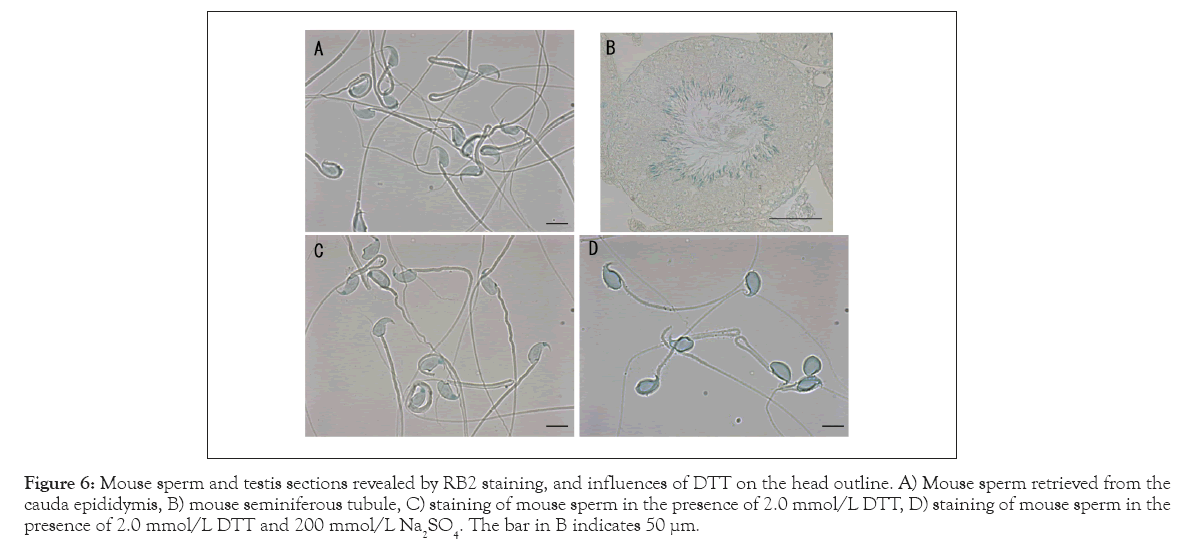
Figure 6: Mouse sperm and testis sections revealed by RB2 staining, and influences of DTT on the head outline. A) Mouse sperm retrieved from the cauda epididymis, B) mouse seminiferous tubule, C) staining of mouse sperm in the presence of 2.0 mmol/L DTT, D) staining of mouse sperm in the presence of 2.0 mmol/L DTT and 200 mmol/L Na2SO4. The bar in B indicates 50 µμm.
At pH 10, RB2 stained the sperm and spermatids (Figure 6), but scarcely stained some cellular components in human semen, the lymphocytes, and the mouse seminiferous tubule (Figures 1,2,4 and 6). The pH-dependent cellular specificity of RB2 was elucidated as follows. The dissociation constants (pk3) of amino and guanidyl residues are 10.5 and 12.5, respectively. Therefore, sulfate residues in RB2 only bind electrostatically with guanidyl residues at pH 10. Approximately 20%-30% of amino acids in histones consist of Lys and Arg, whereas 60–70% of amino acids in protamines consist of Arg. RB2 strongly aggregated with protamines, but not with histones at pH 10. Arg content in the nuclei is related to cellular specificity. Some dyes adsorb to cellular components through various intermolecular forces, such as van der Waals’, hydrophobic, hydrogen, and electrostatic forces. Reactive dyes are used for industrial dyeing of textiles [5], where they adsorb to clothes through chemical bonding. While RB2 belongs to a family of reactive dyes, it is widely known in pharmacology and molecular biology as a potent inhibitor of P2Y purinoceptor and as the ligand for group-specific dye affinity chromatography for albumin, some dehydrogenases, and interferon [10-15]. These properties indicate that RB2 binds with weak intermolecular forces to some cellular components at pH 10, except for its electrostatic binding with Arg in protamines. RB2 helped to visualize not only the tailed sperm but also the spermatids without tails. Severe oligozoospermia may not only decrease the number of sperm, but also increase the number of arrested spermatids (Figure 2D). As shown in Figure 5, the majority of azoospermic semen contained a small number of sperm and spermatids in various phases, which generally gets overlooked under phase-contrast optics. This unique property introduces new aspects to the exploration of spermiogenesis. We must redefine the border between severe oligozoospermia and non-obstructive azoospermia in consideration of spermatids.
Disulfide cross-linking of protamines is essential for the condensation and organization of the shape of the mammalian sperm head [5]. The features of vacuoles in human sperm were heterogeneous (Figures 2 and 3). RB2 staining at pH 10 with DTT led to the complete disappearance of the vacuoles regardless of their original features (Figure 5). On the other hand, the mouse sperm had few vacuoles and maintained their characteristic shape even after treatment with DTT and Na2SO4 (Figure 6). Brykczynska, et al., reported that the aforementioned difference between human and mouse sperm might be related to the approximately 10-fold higher histone content in human sperm than in mouse sperm [16].
Some studies have reported that protamination deficiency is responsible for vacuole formation, and secondarily, for DNA fragmentation [17,18]. The results in Figure 5 indicate that at least in human sperm, local failure of the disulfide cross-linkage may play a critical role in determining the head morphology as well as vacuole formation. The swim-up method could not exclude the motile sperm with vacuoles (Figure 3). Fluorescent staining with SYBR gold revealed vacuoles as low-density areas of DNA [3]. Single-Cell Pulsed-Field Gel Electrophoresis (SCPFGE) suggested that some motile sperm prepared using the swim-up method had already initiated fragmentation [6,19]; SCPFGE could determine whether the fragments were derived from the sperm with vacuoles [20].
In sperm-driven congenital anomalies, the critical threshold of double-strand breaks in a single nucleus is extremely low, and double-strand break beyond the critical threshold causes underutilization and early loss. To clarify whether the failure of cross-linkage is a provocative factor for DNA fragmentation, DNA cleavage at the inner cavity of the vacuoles in the motile sperm needs to be detected. Simultaneous observation of a single sperm using SCPFGE and RB2 staining may be beneficial for drawing a conclusion.
Author has nothing to disclose.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Kaneko S, Okada Y, Yokota S, Takamatsu K (2023) Reactive Blue Dye: Highlights of Vacuoles in Human Sperm. J Med Diagn Meth. 12:400.
Received: 10-Feb-2023, Manuscript No. JMDM-23-21772; Editor assigned: 13-Feb-2023, Pre QC No. JMDM-23-21772; Reviewed: 28-Feb-2023, QC No. JMDM-23-21772; Revised: 10-Mar-2023, Manuscript No. JMDM-23-21772; Published: 20-Mar-2023 , DOI: 10.35248/2168-9784.23.12.400
Copyright: © 2023 Kaneko S et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.