
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Review Article - (2020)
Background: Low Cardiac Output Syndrome (LCOS) is a complication that appears in approximately 20%of cardiac surgeries with extracorporeal circulation. This is associated with increased mortality, delayed recovery and prolonged hospital stay. The Spanish Randomized Clinical Trial on Sindax (SPARTANS) aims to demonstrate the effectiveness of the preoperative use of levosimendan in reducing LCOS in patients with poor Left Ventricle Ejection Fraction (LVEF) undergoing elective cardiac surgery.
Methods: SPARTANS study is a multicenter, randomized triple-blind, placebo-controlled trial. 300 patients with LVEF ≤ 35%, undergoing elective cardiac surgery will be recruited from 9 Spanish hospitals and randomized into two groups: Preoperative administration of levosimendan or placebo for 24 hours. The study drug will be started as a continuous infusion (0.1 μg/kg/ min) at least 8 hours before surgery.
The primary endpoint will be 30-day LCOS. It will be evaluated using any of the following criteria:
1) Postoperated cardiac index ≤ 2.0 L/min/m2, 2) Need to implant a intra-aortic balloon pump/left ventricular assist device, 3) Vasoactive inotropic scale (VIS) >5.5.
The secondary end-point will be composite event rate at one year including the following events: death from any cause, need for renal replacement therapy or dialysis and LCOS.
The sample size is based on the assumption that levosimendan reduces LCOS by 50%.
Conclusion: The effectiveness of levosimendan has not yet been reported with a good evidence in cardiac surgery. We will test the hypothesis that levosimendan reduces LCOS in patients with compromised left ventricular function.
Trial registration number: NCT04179604 (ClinicalTrials.gov).
Rationale and Design of a Spanish Mulicenter Randomized Controlled Trial of Use of Preoperative Levosimendan to Reduce Low Cardiac Output Syndrome (LCOS) in Low Ejection Fraction (≤ 35%) Cardiac Surgery Patients Spanish Randomized Clinical Trial on Sindax (Spartans Study)
LCOS; Poor left ventricule eyection fraction; Levosimendan; Cardiac surgery; Preoperative treatment
Low Cardiac Output Syndrome (LCOS) is defined, by the Working Group of Cardiac Intensive Care of the Spanish Society of Intensive, Critical Medicine and Coronary Units [1], as a cardiac index (CI) <2.2 l/min/m2. LCOS is due to left and/or right ventricular failure and it may be associated with pulmonary congestion and may occur with normal or low blood pressure [2]. LCO after cardiac surgery is associated with increased mortality, delayed functional and organic recovery and prolonged stay in the intensive care unit. It is characterized by a decrease in cardiac function, a reduction in oxygen supply and subsequent tissue hypoxia, together with signs of tissue hypoperfusion (peripheral coldness, confusion, oliguria and elevated lactate level) and in absence of hypovolemia [3].
LCO syndrome appears in approximately 20%of Cardiopulmonary Bypass (CPB) surgeries [4]. Levosimendan, a calcium-sensitizing inotrope and an ATP-sensitive potassium channel opener, has been reported to be effective in decreasing LCOS and mortality after cardiac surgery [5,6]. Simdax, which is its trade name, is indicated for the short-term treatment of severe acute decompensation of chronic heart failure in situations where conventional treatment is not sufficient or in cases where an inotropic support is considered appropriate.
The recent publication of 2 large randomized clinical trials LEVOCTS and LICORN [7,8] failed to meet any benefit of levosimendan in terms of survival in cardiac surgery. Still, in a recently published meta-analysis [9], we showed that Levosimendan is effective in reducing low-cardiac output syndrome compared to placebo (14.8%in the Levosimendan group versus 29.0%in the placebo group; RR=0.40, 95%CI=0.22–0.73; P=0.003). In addition, our meta-analysis showed a decrease in 30-day mortality in patients with moderate or severe dysfunction (RR=0.44, 95%CI=0.27–0.70; P<0.001) and a decrease in dialysis (RR=0.66, 95%CI=0.47–0.92; P=0.015). We could not demonstrate benefit in the reduction of myocardial injury (RR=0.90, 95%CI=0.69–1.17; P=0.44), intensive care unit stay (weighted mean differences=−0.57, 95%CI=−1.15 a 0.01; P=0.055) or the need for ventricular assist device (RR=0.42, 95%CI=0.07-2.63; P=0.35).
These results are consistent with those of the previous 12 metaanalysis carried out on levosimendan used in surgical setting [10-21]. The first 10 of them, which did not include the 2 largest RCTs [7,8], have been systematically reviewed by Pollesello et al. [22]. However the publication of these 2 larges solid trials [7,8] has raised doubts about the usefulness of levosimendan in patients undergoing cardiac surgery.
The purpose of the “Spanish Randomized Clinical Trial on Sindax” (SPARTANS) trial is to evaluate the beneficial effect of preoperative use of levosimendan compared with placebo to reduce perioperative LCOS in patients undergoing cardiac surgery with poor LVF. The main difference of our study to LEVO-CTS and LICORN is that levosimendan will be started earlier before surgery.
Study population
300 patients will be enroll at 9 Spanish University hospitals scheduled for isolated Aortic Valve Replacement (AVR) and/or Coronary Arterry Bypass Grafting (CABG) with Cardiopulmonary Bypass (CPB). All enrolled patients will have a preoperative LVEF equal to, or less than 35%detected by echocardiography measurement one week before surgery.
Eligibility criteria for participation and exclusion criteria are listed in Table 1. All patients are required to give written informed consent, prior to being properly informed about participation in the trial, before enrollment.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age ≥ 18 y | Previous levosimendan administration. |
| Emergency operation. | |
| Kidney or liver trasplant or awaiting it. | |
| Hepatic cirrhosis Child C. In case Child B, contact coordinating center. | |
| Written informed consent | Any degree of preoperative right ventricular failure. |
| LVEF ≤ 35% detected by echocardiography carried out at least one week before surgery. | Preoperative creatinine >2 mg/dl. |
| Valve desease other than aortic. | |
| Renal failure requering dialysis (or creatinine clearance <30ml/min). | |
| Hemodynamic instability (need for inotropics, unstable angina, acute myocardial infarction, intra-aortic balloon pump). | |
| Patients underwent previous cardiac surgery. | |
| Allergy or hypersensitivity to levosimendan or any of its excipients | |
| Severe hypotension (sistolic arterial tension < 80 mmHg or mean arterial pressure <50 mmHg ) and tachycardia ( heart rate >130 bpm). | |
| Scheduled AVR or/and Scheduled CABG with CBP. | History of Torsades de Pointes. |
| Pregnancy or breast-feeding |
Table 1: Inclusion and exclusion criteria.
Study design
The study was approved by the Ethical Committee of all participating centers. SPARTANS is a phase II-III, randomized, triple-blind, placebo-controlled, multicenter clinical trial to evaluate the efficacy of levosimendan in decrease perioperative LCOS in patients whit preoperatively severe left ventricular dysfunction (LVEF ≤ 35%) who will be scheduled for cardiac surgery on CBP. Follow-up duration will be one year. The study design is summarized in Figure 1.
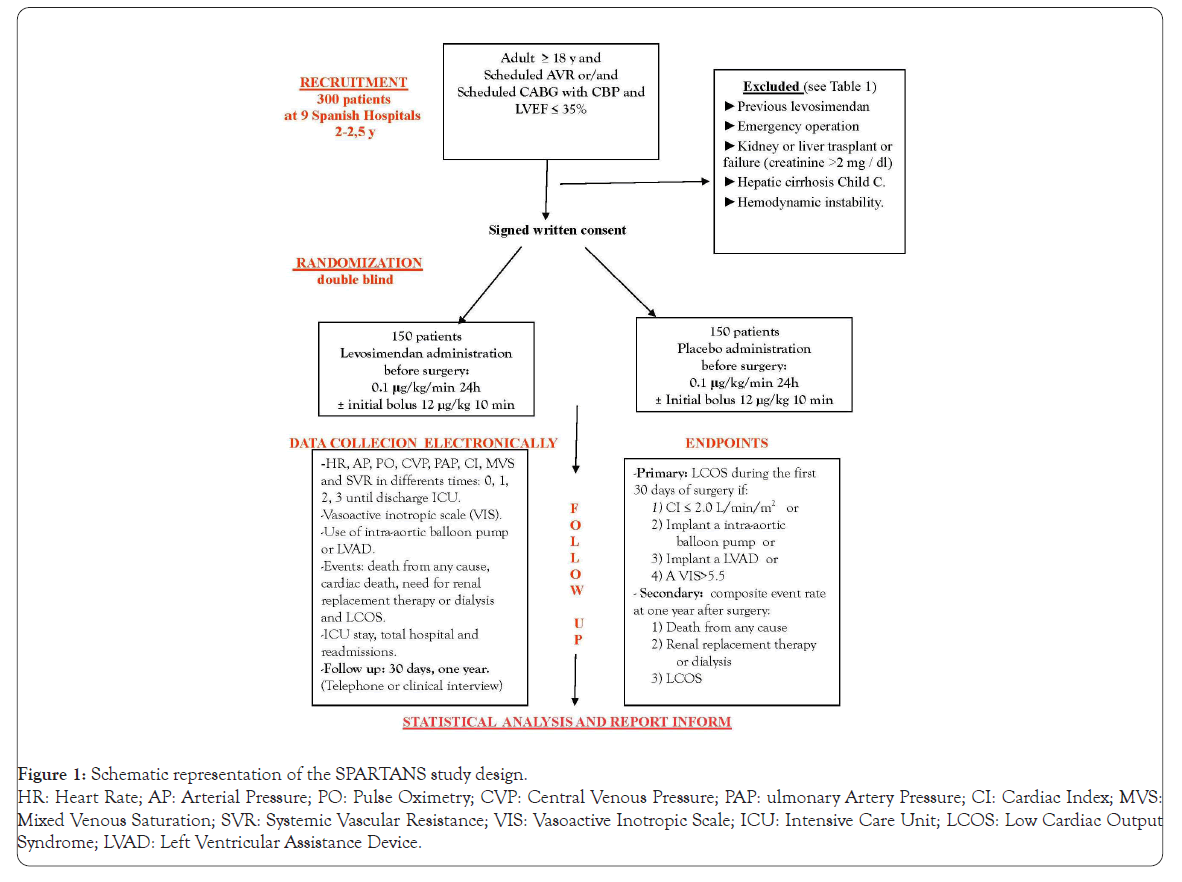
Figure 1: Schematic representation of the SPARTANS study design.
HR: Heart Rate; AP: Arterial Pressure; PO: Pulse Oximetry; CVP: Central Venous Pressure; PAP: ulmonary Artery Pressure; CI: Cardiac Index; MVS:
Mixed Venous Saturation; SVR: Systemic Vascular Resistance; VIS: Vasoactive Inotropic Scale; ICU: Intensive Care Unit; LCOS: Low Cardiac Output
Syndrome; LVAD: Left Ventricular Assistance Device.
Randomization and blinding procedures
We will use a computer-generated permuted block randomization established by an independent statistician. Pharmacy of each hospital will prepare randomized treatment, without being involved in the trial. Patients and physicians will be blind to the randomized therapeutic strategy that each patient will receive. The data will be entered into a specific electronic database in an anonymized and dissociated manner Figure 1.
Study drug administration
Levosimendan SIMDAX 2.5 mg/ml concentrate for solution for infusion. The concentrate is a clear solution, yellow or orange, for dilution before administration. A 5 ml vial contains 12.5 mg of levosimendan. Patients in the placebo group will receive a water-soluble vitamin B2 concentrate with 0.4 mg/ml sodium riboflavin phosphate to obtain the same color as the preparation of levosimendan and ethanol anhydrous 100 mg/ml to resemble the levosimendan odor, which will be administered at the same levosimendan infusion rate. The study drug or placebo infusion will start one day before surgery in an Intensive Care Unit with at least 8 hours of administration before surgery. A continuous infusion at 0.1 μg/kg/min will be administered to complete 24 h duration.
In accordance with current medical practice, hemodynamic monitoring will be carried out during treatment and for at least 3 days after finish infusion or until the patient is clinically stable. In patients with mild to moderate renal or hepatic damage, monitoring will be carried out for at least 5 days, being recorded if adverse events occur.
If the patients once randomized, suffering from one of the following adverse events listed in Table 2 during the infusion, they will be registered stopping the infusion. In the case that the adverse event occurred in the postoperative period and if it was demonstrated due to the cause of the medication under study, it will be registered too.
| Adverse events |
|---|
| Maintained arterial hypotension despite having reduced perfusion by half or having expanded volumetrically. (Systolic arterial pressure <80 mmHg or mean arterial pressure <50 mmHg without response to intravenous fluid therapy or requiring high doses of vasopressors (phenylephrina >100 μg/min, norepinephrine >10 μg/min). |
| Tachyarrhythmias not pharmacologically controlled. Heart rate >130 bpm or <130 bpm with hemodynamic instability. |
| Serious allergic reaction with medication administration. |
| Severe renal or hepatic failure. |
Table 2: Adverse events during levosimendan infusion.
The use of any preoperative concomitant medications, including inotropes and vasopressors, are not indicated because all patients included in the trial have to be hemodynamically stable.
Data collection and Follow up
Data will be collected by a Research Team for each participating hospital that will consist of at least one cardiac surgeon, an anesthesiologist and a specialist in postoperative critical care. These investigators will receive training about patient selection and clinical events registration with the help of Contract Research Organization (CRO TRIDE ASESORES SL). This CRO will carry out regulatory execution of the trial, oversight and collection of electronic data, follow up and scientific regulation of procedures between different ethical Committees and hospitals involved. All data will be evaluated by trial main researcher and CRO identifying possible data collection errors and acting according to good clinical practice.
Patients data will include medical history, cardiac pathology, Euroscore I and II, surgical procedure data including intraoperative mortality as well as hemodynamic data (heart rate, blood pressure, pulse oximetry, central venous pressure, pulmonary artery pressure, cardiac index, mixed venous saturation (SvO2) and systemic vascular resistance). The times will be defined as follows to facilitate data collection:
-Time 0 is defined as the initial hemodynamic measurement, the day before surgery once in an Intensive Care Unit (ICU) before the administration of levosimendan or placebo.
During the administration of levosimendan or placebo, patients will be monitored to detect if an adverse reaction occurs and to act in accordance with the recommendations for withdrawal of subjects(specified in Table 2.
-Time 1 is defined as the hemodynamic measurement immediately after anesthetic induction.
-Time 2 is defined as the hemodynamic measurement collected 20 minutes after CBP.
Time 3 is defined as the hemodynamic measurement registered postoperatively 1 hour after arrival at the intensive care unit.
-From this moment, the data will be collected every 12 hours until discharge from the Intensive Care Unit (ICU).
-If there were the need for inotropics, the Vasoactive Inotropic Scale (VIS) would be calculated: Inotropic Scale (IS)+10 × Milrinone (μg/kg/min)+100 × norepinephrine (μg/kg/ min)+10000 × vasopressin (U/kg/min).
- All serious and nonserious adverse events will be registred and reported to the competent organizations within the established period.
-From the moment of CBP weaning until the 30th day of the surgery, if it would be necessary to use a contra-aortic balloon pump or other type of ventricular assistance device, it will be regirested too.
- Similarly, the following events will be recorded: death from any cause, cardiac death, need for renal replacement therapy or dialysis and low cardiac output syndrome up to one year of follow-up.
-Intensive Care Unit stay, total hospital stay as well as readmissions associated with the intervention will be recorded too.
Once the therapeutic procedure is performed, the Research Team will carry out the clinical follow-up by telephone or clinical interview of the patient according to the time intervals: 30 days and 1 year. We estimate that the total sample size of 300 patients will be reached in 2-2.5 years.
Inotropic and intensive management
Anesthesia, surgery and CBP will be performed according to standard operating procedures and following the usual clinical practice of each hospital. Regarding Inotropic management and weaning of CBP will be performed using an algorithm (Figure 2). The criteria to discharge ICU patients will be: optimal spontaneous ventilation with PaO2>65 mmHg and SpO2>95%; Hemodynamic stability without inotropic support, absence of significant arrhythmias, diuresis without the need for hemofiltration and adequate neurological status.
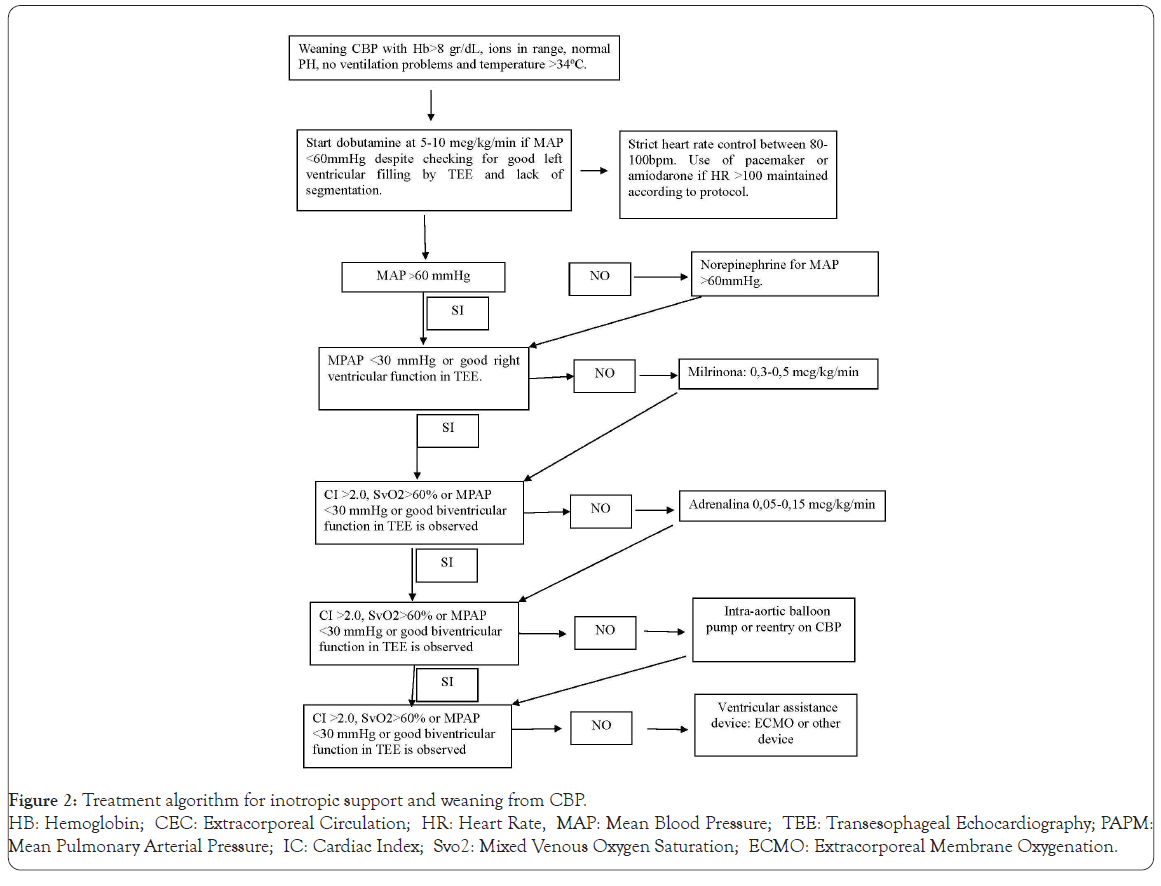
Figure 2: Treatment algorithm for inotropic support and weaning from CBP.
HB: Hemoglobin; CEC: Extracorporeal Circulation; HR: Heart Rate, MAP: Mean Blood Pressure; TEE: Transesophageal Echocardiography; PAPM:
Mean Pulmonary Arterial Pressure; IC: Cardiac Index; Svo2: Mixed Venous Oxygen Saturation; ECMO: Extracorporeal Membrane Oxygenation.
Study endpoints
The primary endpoint is to demonstrate that preoperative levosimendan administration in patients undergoing AVR and/or CABG with poor LVEF ≤ 35%halve perioperative LCOS during the first 30 days of surgery. LCOS will be considered if it meets one of the following criteria:
1) Postoperated cardiac index ≤ 2.0 L/min/m2;
2) The need to implant an intra-aortic balloon pump;
3) The need to implant a left ventricular assistance device;
4) To have a Vasoactive Inotropic Scale (VIS)>5.5*.
*The Vasoactive Inotropic Scale (VIS) will be calculated using the following formula, using a recently published article as a reference [23]: inotropic scale (IS)+10×Milrinone (μg/kg/min)+100 × norepinephrine (μg/kg/min)+10000 ×vasopressin (U/kg/min).
The inotropic scale (SI) will be calculated using the following formula: Dopamine (μg/kg/min)+Dobutamine (μg/kg/min)+100 × adrenaline (μg/kg/min).
The secondary endopoint is: To analyze composite event rate up to one year after surgery that includes the following options:
1) Death from any cause
2) The need for renal replacement therapy or dialysis
3) LCOS
Each component of the secondary composite endpoint will be analyzed separately too. Thus Intensive Care Unit (ICU) stay, total post-surgical stay, hospital cardiac mortality, need for ventricular assistance or intra-aortic balloon pump and need for dialysis will be also collected.
Sample size estimates
The sample size was calculated based on an earlier meta-analysis [9] (Tena MA, et al. Levosimendan versus placebo in cardiac surgery: A systematic review and a meta-analysis. Interact Cardiovasc Thorac Surg. 2018 27: 677-685) where we document that Levosimendan is effective in reducing the rate of low postoperative cardiac output syndrome compared to placebo (14.8%in the Levosimendan group versus 29.0%in the placebo group; RR=0.40, 95%CI=0.22–0.73; P=0.003).
Thus, considering a type I error of 0.05, a type II error of 0.20, a ratio of 1/1, an event rate of 29%in the Placebo group and an event rate of 15%in the Levosimendan group, a sample population of at least 137 patients for each group is required. Assuming a 9.5%patient drop rate, we obtain a total sample of 300 patients (MedCalc Software bvba, Ostende, Belgium, http://www.medcalc. org, 2017).
Thus, 300 patients with LVEF ≤ 35%will undergo cardiac surgery, on CBP, to one of the following procedures: Isolated AVR or CABG or combined (AVR and CABG) being randomized into 2 groups: Levosimendan group (n=150) or Placebo group (n=150).
Statistical analysis
The results will be expressed as means ( ± SD) or as medians and interquartile range to express quantitative variables. Qualitative variables will be expressed by frequency and percentage. Categorical variables will be compared by Fisher's exact test and continuous variables will be compared by Student's test or Mann-Whitney U. Incidence of composite end point will be compared between the two groups using a Chi-square test o Fisher's exact test. The Friedman or Anova test of repeated measures will be used to compare measures repeated through the study time. The risk factors will be expressed through relative risks (ratio of cumulative incidents), Odds Ratio or Hazard Ratio and their respective confidence intervals. Survival curves will be compared using the Log-rank test. All analyzes will be performed 2-sided and with an alpha level of 5%. All analyzes will be performed using the R program, version 3.5.2 or higher (R Foundation). During the third year, the statistical calculation procedure will be carried out.
Study timing, source of financing and conflict of interest
The SPARTANS trial started in December 2019, after Ethics Committee approval, and is raised to finalize recruitment in 2021. The SPARTANS trial is financed by the producer Orion Corporation (Espoo Finlandia) but they will not participate in any phase of the trial. The Principal Investigator is a independent Research. All of the study drugs, both levosimendan and placebo, are provided free of carge from the producer. Statistical analyses will be performed by a experienced statistician. We will follow the recommendations of Helsinki and Consort to carry out the trial and give greater validity to our results. The patients sponsor or researchers included in this trial will not receive financial compensation and declare that there has been no conflict of interest. The SPARTANS trial will end approximately in 2022.
In the last decade several trials have analyzed the impact of levosimendan in cardiac surgery. The results from these published reports have been contradictory, probably because of the inhomogeneous patient selection and study protocols. Moreover, the recent publication of two large randomized clinical trials, LEVO-CTS and LICORN, which failed to meet their primary endpoints of LCOS reduction and had no significant effect on survival, has increased skepticism about levosimendan´s efficacy in cardiac surgery.
In the present study, we aim to utilize the previous data to produce a study design which should utilize the potential benefits of levosimendan in cardiac surgery. The patients have preoperatively low left ventricular ejection fraction. Several meta-analyses have shown that the beneficial effects on survival are seen in those patients and not in those with uncompromised left ventricular function.
Further, the earlier administration of levosimendan in connection to cardiac surgery may give better results than starting the infusion at the induction of anesthesia [24]. In our study, study drug infusion is started 8 to 24 hours before surgery.
The dose of levosimendan will be 0.1 mcg/kg/min, a dose which have did not induce hypotension in LEVO-CTS and LICORN [7,8] as compared to earlier studies with double the dose [25,26].
The aim of the present trial is to give a definitive answer to a simple question: does Levosimendan decrease postoperative low cardiac output syndrome in patients with left ventricle defunction scheduled for cardiac surgery?
A clear answer to this question, due to the dramatic consequences of low cardiac output syndrome in cardiac surgery, will significantly improve the management of patients at risk to suffer from it.
The Spartans trail is articulated on a straightforward protocol, which will recruit only patients scheduled for elective cardiac surgery.
In conclusion, we believe that the present trial will help to give a definitive answer to a very important question.
Citation: Tena MA, Santana L, Urso S, González JM, Fiuza D, Barbeito M, et al. (2020) Rationale and Design of a Spanish Mulicenter Randomized Controlled Trial of Use of Preoperative Levosimendan to Reduce Low Cardiac Output Syndrome (LCOS) in Low Ejection Fraction (? 35%) Cardiac Surgery Patients Spanish Randomized Clinical Trial on Sindax (Spartans Study). J Clin Trials. 10:440.
Received: 04-Nov-2020 Accepted: 17-Nov-2020 Published: 24-Nov-2020
Copyright: © 2020 Tena MA, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.