
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Case Report - (2021)Volume 12, Issue 7
Several Neurologic manifestations have been found in COVID. But there is very little literature on cerebral venous thrombosis association with COVID. We herein report an unusual presentation of patients infected with COVID19 i.e. CVT in 2 patients. We should be aware of this atypical manifestation and early management can have a favourable prognosis. It is prudent to administer vitamin therapy in COVID patients with elevated d-dimer or homocysteine levels due to the risk of CVT. COVID-19 virus is neurotropic and is potentially associated with hypercoagulable state. Neurological symptoms appear either as first symptoms or with respiratory symptoms or anytime during the disease course. Neurological manifestations range from altered sensorium, acute cerebrovascular disease to seizures.
COVID-19; CVT; Hypercoagulability; Stroke; Homocysteinemia
COVID-19: Corona Virus Disease of 2019; CVT: Cerebral Vein Thrombosis; GCS: Glasgow Coma Scale; CBNAAT: Cartridge Based Nucleic Acid Amplification Test; NCCT: Non-contrast Chest Computed Tomography; SAH: Subarachnoid Haemorrhage; CECT: Contrast Enhanced Computed Tomography; HRCT: High Resolution Computed Tomography; ICU: Intensive Care Unit; DM: Diabetes Mellitus; MTHFR: Methylenetetrahydrofolate Reductase; CNS: Central Nervous System
COVID-19 virus is neurotropic and is potentially associated with hypercoagulable state. Neurological symptoms appear either as first symptoms or with respiratory symptoms or anytime during the disease course. Neurological manifestations range from altered sensorium, acute cerebrovascular disease to seizures. One of the rare manifestations is cerebral venous thrombosis (CVT) and there are very few isolated reports of CVT associated with COVID-19 [1,2]. We herein report a unique series of 2 cases of CVT associated with COVID-19 from a single academic hospital. Awareness about this rare manifestation and timely anticoagulation is of utmost importance for prevention of catastrophic events like death. Simple additional measures can be beneficial for its management, which have been discussed. Written informed consent was taken to use patient information after deidentification.
Case 1
A 65- year old (50 kg) female presented to our emergency department with history of altered sensorium and 3 episodes of seizures since one day. She had medical history of diabetes and hypertension (irregular medication) was referred to our COVID center, after 5 days of treatment in other hospital.
On examination she had altered sensorium with GCS of 9 (E3V1M5), left hemiparesis and was hemodynamically stable maintaining oxygen saturation on room air. The nasopharyngeal swab specimen confirmed the diagnosis of COVID-19 by Cartridge Based Nucleic Acid Amplification Test (CBNAAT). On admission the non-contrast chest Computed Tomography (NCCT) showed right frontal lobe infarct and right fronto-parietal subarachnoid haemorrhage (SAH) with mild cerebral edema in Table 1.
| Cases of COVID-19 Symptoms | Case 1 | Case 2 |
|---|---|---|
| Median time from COVID-19 symptoms to a thrombotic event | 5 days | 1 day |
| Associated Comorbidities | Diabetic and hypertensive on irregular medication | No |
| Any risk factor: Smoking, dehydration, OCP, Diabetes mellitus, systemic inflammatory disease, cancer, hematologic disorders, head and neck infections, and CNS infections and hyperhomocysteinemia, anemia, family history | Diabetes mellitus, systemic inflammatory disease, | Smoking, hyperhomocysteinemia |
| Signs of dehydration at admission | No | No |
| Sinus involved | Superior sagittal sinus, right transverse, sigmoid sinus and internal jugular vein | Superior sagittal sinus |
| Medical management | Methylprednisolone, Doxycycline, piperacillin tazobactam, Inj enoxaparin BD | Doxycycline, piperacillin tazobactam, Inj enoxaparin BD |
| Anti-edema measures | Mannitol and lasix, seizure prophylaxis with phenytoin and levetiracetam | Mannitol and lasix, levetiracetam |
| Clinical course | Succumbed to multiorgan failure | Good |
Table 1: A Brief overview of the two cases of COVID 19 with cerebral venous thrombosis.
She was admitted to intensive care unit (ICU) maintaining oxygenation on a simple facemask at 5 litres/min of oxygen and had a PO2 of 223 mmHg. She started on anti-edema measures (mannitol and furosemide), seizure prophylaxis (phenytoin and levetiracetam) and anti COVID supportive therapy (methylprednisolone 40 mg bd, doxycycline 100 mg bd). Her GCS improved to 14 (E4V4M6) in the next few hours. She had raised total leukocytes (20600/mm), inflammatory markers (IL6, Ferritin: 42.88 pg/ml and 534.9 ng/ ml) and raised D-dimers (>20 mcg/ml) initially. Later on CECT brain revealed dural venous sinus thrombosis involving superior sagittal sinus, right transverse, sigmoid sinus and internal jugular vein with venous infarcts in bilateral frontal lobes with hemorrhage and diffuse SAH (Figure 1a-1d). In consultation with neurology, she was started on Inj enoxaparin 60 mg bd. On the third day of ICU admission her GCS deteriorated to 7 (E1V1M5) and she became hemodynamically unstable and she was intubated. During the course of her admission, her oxygenation need was FiO2 40%-50% and had worsening of the clinical parameters necessitating ionotropic requirement of noradrenaline and vasopressin. Repeat serial CT brain revealed the same findings with no change in the hematoma or SAH. Ultimately despite high end antibiotics, she developed septic shock and multiple organ failure (Thrombocytopenia, renal failure). On the 7th day of ICU admission, her GCS dropped to (E1VETM2) but had no change in neuroimaging. There was loss of brainstem reflexes and fixed dilated pupils by day 10 with persistent septic shock and a new intracranial bleed (Figure 1b) due to thrombocytopenia. She succumbed to multiorgan failure. Unfortunately, throughout her course we could not diagnose a specific cause of her cerebral venous thrombosis barring COVID/sepsis related disseminated intravascular coagulation.
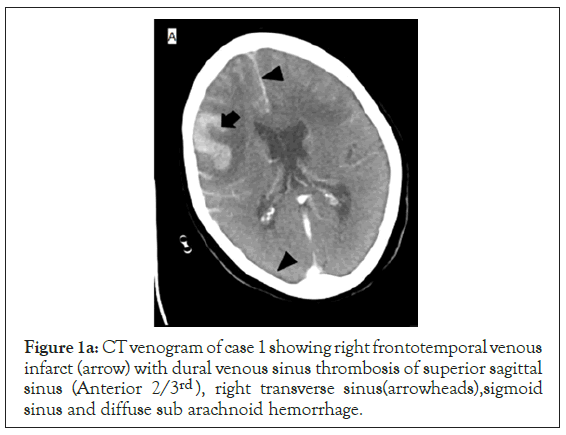
Figure 1a: CT venogram of case 1 showing right frontotemporal venous infarct (arrow) with dural venous sinus thrombosis of superior sagittal sinus (Anterior 2/3rd ), right transverse sinus(arrowheads),sigmoid sinus and diffuse sub arachnoid hemorrhage.
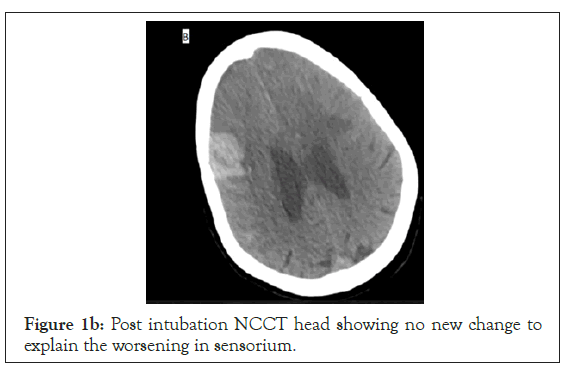
Figure 1b: Post intubation NCCT head showing no new change to explain the worsening in sensorium.
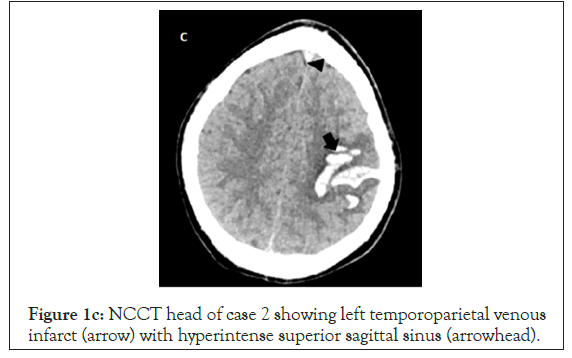
Figure 1c: NCCT head of case 2 showing left temporoparietal venous infarct (arrow) with hyperintense superior sagittal sinus (arrowhead).
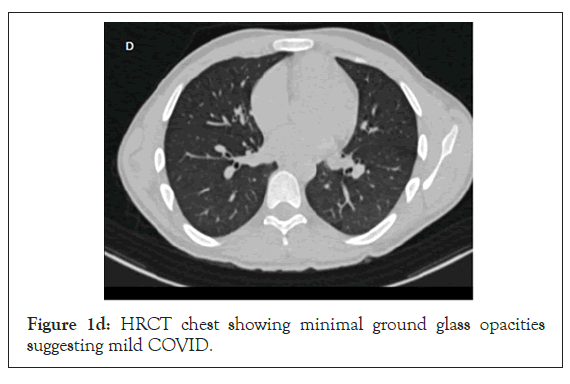
Figure 1d: HRCT chest showing minimal ground glass opacities suggesting mild COVID.
Case 2
A 19- year old (60 kg) male with no comorbidities presented with Right sided hemiparesis (upper limb power 2/5, lower limb power 3/5) and 1 episode of seizure at the time of presentation, with a GCS of 15. His non contrast CT head showed left frontal/ parietal bleed and a hyper dense superior sagittal sinus suggestive of thrombosis (Figure 1c). His HRCT chest was suggestive of mild COVID (Figure 1d) and he didn’t require oxygen therapy.Even his inflammatory markers (IL6 and ferritin) were low 28.59,27. His GCS remained 15/15 throughout his hospital stay. He was managed with antiedema measures (mannitol), seizure prophylaxis (Inj. levetiracetam 1 g BD), and Inj enoxaparin 60 mg BD for his cerebral venous thrombosis. As anti-COVID he received the standard management as above, barring the steroids. During the course of treatment, he developed one episode of generalized tonic clonic seizures on 4th day of ICU stay which was aborted with midazolam iv 2 mg and the dose of levetiracetam was increased to 1.2g BD. Repeat NCCT head showed no new hemorrhage. His blood work showed an increased homocysteine level of 126 micmol/L which was 8.4 times the upper limit of normal, coupled with a low vitamin B12 of 100 pg/ml (N:197-771 pg/ml). He was started on injectable vitamin B12, Tab Folic acid and Tab Pyridoxine. He was later bridged over to warfarin.
We herein report an unusual presentation of patients infected with COVID19 i.e CVT in 2 patients. CVT has been linked to various hypercoagulable states which include oral contraceptives, pregnancy, puerperium, DM, malignancy, systemic inflammatory disease, cancer, hyperhomocysteinemia, anemia, hematologic disorders, head and neck infections, CNS infections and viral infections. Heritable thrombophilia’s like antithrombin, protein C and S deficiency may attribute to CVT but it might not be feasible to get these levels in all hospital settings [3]. Viral infections are known to cause endothelial dysfunction potentiating thrombin formation and additionally hypoxemia can raise the blood viscosity and activate hypoxia related genes mediating coagulation and thereby thrombotic events [4,5]. Patient 1 had DM as risk factor while patient 2 had smoking as the risk factor besides COVID infection.
Several Neurologic manifestations have been found in COVID which include central nervous system manifestations (dizziness, headache, impaired consciousness, acute cerebrovascular disease, ataxia, confusion, hallucinations and seizure, encephalopathy, encephalitis and cerebrovascular pathologies, acute myelitis, and Guillain-Barre syndrome), peripheral nervous system manifestations (gustatory impairment, olfactory dysfunction, vision impairment, and nerve pain), and skeletal muscular injury manifestations [6,7]. However, there is very little literature on CVT association with COVID.
COVID-19 infection elevates homocysteine levels which are synthesized from methionine by methylenetetrahydrofolate reductase (MTHFR). Raised homocysteine levels activate the coagulation cascade increasing D-dimer production. Collectively, there is increased formation of blood clots leading to severe complications like CVT. It has been suggested that there is molecular connection between some genes and D-dimer and homocysteine levels during coagulation [8].
We were unable to work up the homocysteine levels and other causes of a prothrombotic state due to rapid deterioration in patient 1 but in patient 2 there were high homocysteine levels and low folate levels. It is still not well established whether SARS- CoV-2 induces a prothrombotic state with hyperhomocysteinemia as the major mechanism or one of the multiple mechanisms for hypercoagulability.
Low levels of serum folate, pyridoxine, and cobalamin are associated with hyperhomocysteinemia which in turn are associated with associated with an increased risk of CVT [3]. Hence, it is prudent to administer vitamin therapy in patients with elevated d-dimer which is a consistent finding in CVT. Besides, vitamin therapy (Folate , pyridoxine and cobalamin) has the potential role to reduce the risk of recurrence of CVT in non covid patients [3].
Management includes reversal of the underlying cause, control of seizures and intracranial hypertension, and prompt anticoagulation. Prompt anticoagulation was started in both the patients but patient 1 had CVT of multiple sinuses with SAH, presented late and her clinical condition deteriorated very rapidly and succumbed to multiorgan failure. Patient 2 was young, had single sinus involvement and early administration of anticoagulation together with treating the cause (raised homocysteine, folate deficiency) resulted in favorable outcome.
We should be aware of this atypical manifestation and adequate management can have favourable prognosis. Vitamin therapy can be considered in COVID patients with elevated d-dimer or homocysteine levels due to the risk of CVT.
Citation: Patel N, Aggarwa R, Ganesh V, Kaur M, Soni KD, Trikha A (2021) Rare Manifestations of Dreaded COVID-19 Cerebral Venous Thrombosis: A Case Report. J Anesth Clin Res. 12:1008.
Received: 10-Jun-2021 Accepted: 24-Jun-2021 Published: 01-Jul-2021 , DOI: 10.35248/2155-6148.21.12.1008
Copyright: © 2021 Patel N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.