Angiology: Open Access
Open Access
ISSN: 2329-9495
ISSN: 2329-9495
Research Article - (2019)Volume 7, Issue 2
Aim: In the present study, we analysed the learning curve of prosthetic vascular access creation. Materials and methods: The first 50 consecutive prosthetic vascular access created by a single experienced vascular surgeon was included in this study. Primary outcomes were operative time, intervention-free access survival and functional access survival. Additional outcomes were complications of the intervention. We used the cumulative sum technique to assess the learning curve.
Results: The analysis of the learning curve obtained with cumulative sum technique on operative time, interventionfree survival and functional access survival permitted to define three phases: learning (first 25 patients), expertise (following 15 patients) and post-learning phase. Accordingly, statistical differences were observed in operative time, intervention-free survival, and frequencies of graft thrombosis among the three groups.
Conclusion: Prosthetic vascular access creation is a safe and effective intervention when performed by an experienced vascular surgeon. Otherwise, a twenty-five interventions learning curve is mandatory to obtain better results in terms of operative time, circuit survival and frequency of thrombosis. Additional fifteen interventions are required to obtain an expert level of practice, allowing for more challenging procedure.
Cloning, Stem cells, Treatment, Moral problems
AF: Atrial Fibrillation; AMI: Acute Myocardial Infarction; AAVS: American Association for Vascular Surgery; aVA: autogenous Vascular Access; COPD: Chronic Obstructive Pulmonary Disease; CUSUM: cumulative sum; CVC: Central Vein Catheter; ESKD: End-Stage Kidney Disease; FAS: Functional Access Survival; KDOQI: Kidney Disease Outcomes Quality Initiative; IFAS: Intervention-Free Access Survival; LC: Learning Curve; NKF: National Kidney Foundation; OT: Operative Time; PAD: Peripheral Arterial Disease; PD: Peritoneal Dialysis; pVA: prosthetic Vascular Access; SVS: Society for Vascular Surgery
Kidney transplant represent the definitive treatment for End-Stage Kidney Disease (ESKD). However, the availability of grafts remains the main limit of this therapy. Accordingly, hemodialysis is the most widely used treatment for ESKD, and represents a life-saving procedure for urgent situation. Vascular access is a key component for hemodialysis in those patients who are waiting for graft or for those not candidate to kidney transplantation [1]. Nevertheless, obtaining a functioning vascular access is not always easy. In fact, the failure of autogenous Vascular Access (aVA) maturation depends on many, and mostly unknown, factors [2]. Prosthetic Vascular Access (pVA) could represent a valuable alternative to aVA in case of maturation failure, thrombosis or poor vascular network [3]. Additionally, the introduction of new prosthetic grafts especially designed for early cannulation allowed pVA to become a suitable alternative to Central Venous Catheters (CVC) in urgency and emergency [4]. The increasing use of these devices must face a surgeon’s multidisciplinary preparation. For this reason, gaining experience with pVA surgery may not be simple even for an experienced vascular surgeon. A learning curve is a mathematical and geometrical representation of a learning process not only consequent to the repetition of surgical gesture, but also needing the comprehension of pathophysiological processes. Vascular access surgery requires achieving many technical competences and a background of hemodynamic and dialysis knowledge. The focus of the present paper is to evaluate surgeon’s operative competency based on different parameters such as operative time, complications, intervention-free graft survival and functional access survival.
Arteriovenous fistulas are the gold standard for vascular access in patients with end-stage kidney disease requiring hemodialysis because of the better primary patency and the low complication rate [1]. The pVA represents a valid alternative especially for an exhausted or failed access on native vessels, or when vessels are not suitable [5,6]. Furthermore, many authors underlined the usefulness of pVA in urgency or emergency as bridge instead of central line placement in patients with future suitable autogenous alternatives [4]. In fact, complications and increased risk of death associated to central vein catheters have been recently pointed by many authors [7,8].
Creation of vascular accesses on native vessels is part of the technical background of many nephrologists and surgeons: this is not always true for the prosthetic alternative. In fact, it requires specific technical knowledge, a suitable operating room and appropriate anesthesiologic support. Considering the need of these specific settings, pVA creation is commonly a vascular surgery prerogative. In this scenario proper surgery skills, familiarity with hemodynamics, cannulation and dialysis management are required. As a consequence, proper training on pVA surgery and its care must be taken in count. In fact, it would be a mistake for the experienced vascular surgeon to consider pVA creation a conjugation of what is routinely done in peripheral vascular surgery. As previously reported by Davidson et al., medical education and training in dialysis access is complex and inadequate.
Main reasons are represented by:-
1. Heterogeneity in background of physicians and surgeons involved in vascular access creation
2. Absence of a specific training
3. Misunderstanding about best educational model [9]
It happens that vascular access practice is left on the side-lines of other surgical activities considered more prestigious. Accordingly, the training of vascular surgeons often does not involve the practice on vascular accesses. In this regard, it is noteworthy how literature stressed the importance of training on alternative models such as for example simulated models [10]. Unfortunately, even the present paper considered the opportunity of including a proper training on simulators assessing possible benefit.
Notably, vascular access surgery includes knowledges and skillbased algorithms regarding when and how to use new and proper technologies, frequently expensive and potentially dangerous for patients [11]. Accordingly, Davidson et al. suggested a new paradigm of future dialysis access training in order to accommodate learners with different individual and professional backgrounds, and proper skill development [12]. More recently, Edwards et al. described their experience with a new modular vascular access training program for surgical trainees [13]. Many other authors presented their experience in vascular access training demonstrating how vascular surgeon expertise affect the immediate and long-term outcome of newly created vascular accesses. For example, Gifford et al. showed an overall improvement in technical ability (in terms of procedure time, p<0.001; technical errors p=0.03) in association to the number of procedures performed by surgeon residents. However, the authors did not demonstrate a statistical correlation considering the single surgeon resident [14]. Regus et al. demonstrated an increased risk of aVA immediate failure (p=0.005) or reduced primary patency (p<0.001) whenever the fistula was created on the forearm by unexperienced surgeons [15]. Unexpectedly, we did not find any previous paper on learning curve for pVA creation. Literature suggests many approaches for LC evaluation and plotting. Statistical process-control methods such as the CUSUM are well-known examples [16,17]. The cumulative sum technique was originally developed by industries to monitor productive performance and quality [18]. In the Seventies, it was adopted in medical statistics to analyze surgery-related learning curves. LC were firstly used in pediatric cardiac surgery [19], and more recently in other surgical fields namely laparoscopic and urologic surgery [20,21].
Study design and settings
The present work is a retrospective observational cohort study over a single centre routine surgical practice. Data were collected prospectively. The study includes the first newly-created 50 Prosthetic Vascular Access graft (pVA) by a single experienced vascular surgeon. A detailed description of pVA indication and surgical technique has already been provided [3]. Exclusion criteria are previous history of complicated vascular access, and/or completion additional procedures (e.g. fistula ligation, thrombectomy, angioplasty).
To assess the Learning Curve (LC) we included Operative Time (OT), and pVA survival defined by Intervention-Free Access Survival (IFAS) and Functional Access Survival (FAS). Additionally, patient outcomes such as mortality, frequency of complications and central line placement were considered.
Evaluation and follow-up
All the patients were followed for 1800 days after intervention. Patients underwent routine clinical and Doppler ultrasound examination twice a month. Complications and/or additional procedures were regularly recorded and tabulated in a dedicated database. Surgical indications to pVA additional procedures or re-interventions for complications were evaluated according to National Kidney Foundation (NKF) Kidney Disease Outcomes Quality Initiative (KDOQI) [22]. All procedures were performed at our center (Figure 1).
Figure 1: Kaplan-Meier survival function over 1800 days. Comparison of Intervention-Free Access Survival (IFAS) and Functional Access Survival (FAS) based on the three phases of the operation time learning curve: phase 1 (green), phase 2 (blue), phase 3 (orange).
Definitions and outcomes criteria were defined according to the Committee on Reporting Standards of the Society for Vascular Surgery and the American Association for Vascular Surgery (SVS/AAVS) on vascular accesses [23].
CUSUM analysis
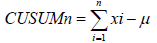
Learning curve was assessed with Cumulative Sum (CUSUM) control chart technique. The CUSUM is a recursive function given by the cumulative sum of differences between single data points (xi) and the mean (μ) of all data [8,17,20,24].
As suggested by Park et al., after ordering data chronologically, CUSUM is calculated for each indicator as the difference between the value of the first case and overall mean value. CUSUM of the second and consequent cases were the previous case’s CUSUM added to the difference between the value of each case and overall mean value. This recursive process continued sequentially to the last case. Finally, CUSUM was plot as line chart, and data were modelled as polynomial. Trend line inflections were analysed in order to define changes in LC. Generally, LC presents three expected phases:
1) Positive inclined line during initial learning curve
2) Plateau during additional experience obtainment
3) Decline of the curve in the post-learning period [21].
Statistical analysis
Continuous variables are reported as mean ± standard deviation (range: minimum–maximum). For counts and categorical data, frequencies are reported with percentage in parentheses. T test was used to compare continuous variables and chi-square test for categorical. Kaplan-Meier estimator was used to assess access survival, and Log Rank to compare survival curves. SPSS software version 24.0 (SPSS Inc, Chicago, IL) was used for statistical analysis. All statistical tests were two-tailored, and p-value <0.05 was considered to indicate statistical significance (Figure 1) (Tables 1A and 1B).
Table 1A: Intervention-Free Access Survival (IFAS), Log Rank p=0.024
| Intervention-free access survival (IFAS) | ||||
|---|---|---|---|---|
| 6 months | 12 months | 24 months | 48 months | |
| Phase 1 | 80.00% | 60.00% | 26.70% | 20.00% |
| At risk | 19 | 13 | 4 | 1 |
| Phase 2 | 95.80% | 87.10% | 57.70% | 32.10% |
| At risk | 14 | 12 | 6 | 2 |
| Phase 3 | 100% | 85.70% | 71.40% | 57.10% |
| At risk | 10 | 7 | 6 | 5 |
Table 1B: Functional Access Survival (FAS), Log Rank p=0.586
| Functional access survival (FAS) | ||||
|---|---|---|---|---|
| 6 months | 12 months | 24 months | 48 months | |
| Phase 1 | 93.30% | 93.30% | 79.00% | 57.40% |
| At risk | 22 | 20 | 17 | 8 |
| Phase 2 | 95.70% | 91.30% | 71.70% | 66.20% |
| At risk | 14 | 13 | 9 | 7 |
| Phase 3 | 88.90% | 88.90% | 76.20% | 76.20% |
| At risk | 9 | 8 | 8 | 7 |
Fifty consecutive patients underwent pVA creation between July 2011 and May 2014. Eighteen (36.0%) were male. Mean age was 64 ± 12 years (range: 42-87). Demographic characteristics are reported in Table 2. Graft implantation was technically successful in all cases. No intra-operative thrombosis was observed. Mean surgery time was 72 ± 15 minutes (range, 55-140). Blood loss was 50 ± 16 mL (range, 20 -150). In-hospital mortality was not observed; major local or systemic morbidity was not observed. Mean hospitalization was 3 ± 1.75 days (range, 1- 4). Patency of the pVAs was 100% at discharge. No patient was lost during follow-up and follow-up index was 1.0. Cannulation of the pVA was performed after wound healing: mean latency between pVA implantation and cannulation was 10 ± 5 days (range, 1-21). Six (12.0%) patients did not start haemodialysis through the graft cannulation for the following causes: arm swelling (n=3), haematoma (n=2), skin infection (n=1). Complications were treated conservatively but a central line was always required.
Table 2: Demographic data and risk factor.
| Characteristics of the study population and vascular access | |
|---|---|
| Study population N=50 (%) |
|
| Demographic data | |
| Age, years ± SD (range) | 64 ± 12 (42-87) |
| Male | 18 (36) |
| Female | 32 (64) |
| Comorbidities | |
| Hypertension | 36 (72) |
| AMI | 14 (28) |
| Diabetes | 16 (32) |
| AF | 7 (14) |
| COPD | 8 (16) |
| Autoimmune disease | 3 (6) |
| PAD | 16 (32) |
| Haemodialysis | |
| Previous aVA | 24 (48) |
| CVC | 12 (24) |
| Non in dialysis | 10 (20) |
| PD | 4 (8) |
| Reason for pVA | |
| Proximal aVA malfunctioning | 24 (48) |
| Inadequate vessels | 11 (22) |
| CVC infections | 7 (14) |
| CVC malfunction | 5 (10) |
| Crash lander or inadequate planning | 3 (6) |
| pVA characteristics | |
| Right arm | 11 (22) |
| Left arm | 39 (78) |
| Radio – basilic | 27 (54) |
| Brachial – Basilic | 14 (28) |
| Radio – Cephalic | 5 (10) |
| Brachial – Cephalic | 4 (8) |
| N: Number; SD: Standard Deviation; AMI: Acute Myocardial Infarction; AF: Atrial Fibrillation; COPD: Chronic Obstructive Pulmonary Disease; PAD: Peripheral Arterial Disease; PD: Peritoneal Dialysis; aVA: autogenous Vascular Access; pVA: prosthetic Vascular Access; CVC: Central Vein Catheter | |
Eleven (22.0%) patients died during the follow-up period. Causes of death were not related to pVA.
Mean time between pVA creation and death was 397 ± 350 days (range 25-1027). Only three patients died within the first 6 months after intervention and causes of death were cerebral haemorrhage (n=1), intestinal perforation (n=1) and trauma (n=1).
Thirty-eight access thrombosis occurred in 23 (46.0%) patients. Nine (23.7%) early thrombosis within the first six postoperative months were observed in 9 (39.1%) patients. Among them, causes of thrombosis were outflow stenosis (n=3), severe hypotension (n=2), autonomous decision to suspend the dual antiplatelets therapy (n=1). In the remaining 3 cases technical problems are plausible. Twenty-nine (76.3%) remaining access thrombosis occurred in 14 patients. In particular, two patients had three events, seven patients had two events, and the remaining nine patients only one. In all cases a stenosis was detected and treated with anastomosis redo (n= 9), vein angioplasty (n=20) and completion endograft stenting (n=4). Intervention-free access survival was 79.6%, 50.9%, 34.0% at 12, 24 and 48 months respectively. Prosthetic vascular access failure occurred in 17 (34.0%) cases and due to extensive graft damage for repeated cannulation (n=8, 16.0%), recurrent thrombosis (n=5, 10%), hematoma or graft infection after wrong cannulation (n=4, 8.0%). Functional access survival was 91.1%, 76.6%, 66.2% at 12, 24 and 48 months respectively. Follow-up complications are resumed in Table 3.
Table 3: Differences in operative time and number of thrombosis are statistically significant among the three phases of learning curve.
| Risk factors and complications among the three phases of learning curve | ||||
|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 3 | P | |
| N=25 (%) | N=15 | N=10 | ||
| Demographic data | ||||
| Age, years ± SD (range) | 62 ± 12 (42-85) | 65 ± 12 (52-84) | 66 ± 14 (42-84) | 0.766 |
| Male | 9 (36) | 5 (33.3) | 4 (40.0) | 0.715 |
| Female | 16 (64) | 10 (66.7) | 6 (60.0) | 0.709 |
| Death | 6 (24) | 3 (20) | 2 (20) | 1 |
| Comorbidities | ||||
| Hypertension | 20 (80) | 9 (60) | 7 (70) | 0.273 |
| Diabetes | 8 (32) | 5 (33.3) | 3 (30) | 1 |
| PAD | 9 (36) | 4 (26.7) | 3 (30) | 0.73 |
| AMI | 8 (32) | 4 (26.7) | 2 (20) | 0.728 |
| AF | 4 (16) | 2 (13.3) | 1 (10) | 1 |
| COPD | 4 (16) | 3 (20) | 1 (10) | 1 |
| Autoimmune disease | 2 (8) | 1 (6.7) | 0 | 1 |
| Haemodialysis | ||||
| Previous aVA | 11 (44) | 7 (46.7) | 6 (60) | 0.471 |
| CVC | 7 (28) | 4 (26.7) | 1 (10) | 0.391 |
| Non in dialysis | 5 (20) | 3 (20) | 2 (20) | 1 |
| PD | 2 (8) | 1 (6.7) | 1 (10) | 1 |
| Reason for pVA | ||||
| Proximal aVA malfunctioning | 11 (44) | 7 (46.7) | 6 (60) | 0.472 |
| Inadequate vessels | 5 (20) | 4 (26.7) | 3 (30) | 0.661 |
| CVC infections | 5 (20) | 2 (13.3) | 0 | 0.291 |
| CVC malfunction | 2 (8) | 2 (13.3) | 1 (10) | 0.623 |
| Crash lander or inadequate planning | 2 (8) | 1 (6.7) | 0 | 1 |
| pVA characteristics | ||||
| Operative time | 97 ± 60 (55-140) | 61 ± 5 (58-72) | 56 ± 6 (55-62) | 0.027 |
| Left arm | 19 (76) | 14 (93.3) | 6 (60) | 0.224 |
| Radio – basilic | 15 (60) | 8 (53.3) | 4 (40) | 0.453 |
| Brachial – Basilic | 5 (20) | 7 (46.7) | 2 (20) | 0.091 |
| Radio – Cephalic | 2 (8) | 2 (13.3) | 1 (10) | 0.623 |
| Brachial – Cephalic | 3 (12) | 0 | 1 (10) | 0.278 |
| Complications | ||||
| Thrombosis | ||||
| Patients | 12 (48) | 8 (53.3) | 3 (30) | 0.458 |
| Events | 21 (55.3) | 10 (26.3) | 7 (18.4) | 0.002 |
| Infections | 3 (12) | 0 | 1 (10) | 0.4 |
| CVC placed | 4 (16) | 1 (6.7) | 1 (10) | 0.633 |
| N: Number; SD: Standard Deviation; AMI: Acute Myocardial Infarction; AF: Atrial Fibrillation; COPD: Chronic Obstructive Pulmonary Disease; PAD: Peripheral Arterial Disease; PD: Peritoneal Dialysis; aVA: autogenous Vascular Access; pVA: prosthetic Vascular Access; CVC: Central Vein Catheter | ||||
Cumulative sum learning curve (Figure 2) for OT, IFAS, and FAS were better modelled as a third-order polynomial with a relatively high coefficient of determination (0.41>R²<0.73 ). As expected, OTrelated learning curve consisted of 3 phases: phase 1 (first 25 cases), phase 2 (subsequent 16 cases), phase 3 (last 9 cases). Comparisons between the 3 phases identified by the OT CUSUM analysis are presented in Table 3. We did not find statistical differences in terms of demographic data and type of pVA configuration. Otherwise, thrombosis and operative time showed a statistically significant decrease in phase 2 (thrombosis: p=0.019; operation time: p=0.027) and phase 3 (thrombosis: p=0.002; operation time: p=0.040) in comparison to phase 1. Additionally, interventionfree- survival was statistically higher in group 2 and 3 compared to group 1 (p=0.024, Figure 1A). Although functional access survival was higher in groups 2 and 3 (Figure 1B), this difference was not statistical significative (p=0.586).
Figure 2: Cumulative Sum (CUSUM) of Operative Time (OP) [A], Intervention-Free Access Survival (IFAS) [B], Functional Access Survival (FAS) [C].
A. Overall operative time (blue) plotted against cumulative sum (orange); the discontinue line represent the best fit curve for the CUSUM plot, which is a third-order polynomial with equation CUSUMOT=-0.0006×case number 3+0.05×case number2– 5×case number+25.1 (R²=0.60).
B. Overall intervention-free access survival (blue) plotted against cumulative sum (orange); the discontinue line represent the best fit curve for the CUSUM plot, which is a third-order polynomial with equation CUSUMIFAS=-0.04 × case number 3+0.05×case number 2+ 83.5×case number-252.3 (R²=0.41).
C. Overall functional access survival (blue) plotted against cumulative sum (orange); the discontinue line represent the best fit curve for the CUSUM plot, which is a third-order polynomial with equation CUSUMFAS=0-0.5×case number 3+37.2×case number 2–658.4×case number+2100.6 (R²=0.73).
We reported in Figure 1 CUSUM learning curves related to OT, IFAS and FAS. We noticed that while FAS curve consisted of 3 phases, IFAS curve presented an initial negative tendency. Nevertheless, we considered it part of the first phase as described later.
In the initial LC phase, after 25 patients, a trained vascular surgeon with no prior experience in pVA creation reaches full competence in all the parameters analyzed. After 15 more cases the surgeon achieves expert abilities. As observed, overall operative time and occurrence of thrombosis show a decrease after the first phase. Overall IFAS and FAS are satisfactory, and in line with literature. However, expected intervention-free survival increased in phase 2 and 3. Otherwise, functional access survival did not show a solid statistical association to our LC model. In our opinion, it could be explained with the fact that complications leading to graft definitive failure in our series were largely outside the surgeon's responsibility. In fact, 70.5% of graft abandonment was due to graft waste or wrong cannulation. Furthermore, the negative trend characterizing phase 1 of FAS plot could be explained with a bad management and wrong cannulation of the new device at the beginning of haemodialysis session by inexperienced nurses. On the other hand, stenosis and consequent thrombosis can be considered under the surgeon control. In fact, although not clearly demonstrated, graft-to-vein anastomosis geometry and distance from valves may afflict the patency of the circuit [25,26].
The present paper seems to confirm previous observations conducted on aVA training programs [9–15]. Nevertheless, there are many limitations affecting the present paper. Firstly, its retrospective design: although data were prospectively collected, it may include potential confounding variables. Despite the fact that no specific methods were used in order to reduce selection bias, we have to notice that demographic data and graft configurations have been well-matched. Additionally, no specific phase included cases technically challenging, or, on the contrary, particularly easy, especially at the beginning of the curve. Secondly, a single center experience may suffer pros and cons of a multidisciplinary care protocol, not fully implemented in every hospital. Many studies stressed the relevance of surgeon’s custom and familiarity with diagnostic protocols and modern technologies, especially during the follow-up and graft recue [27-30]. Finally, we considered a single-surgeon experience with a single graft. On one hand this can be considered a standardizing factor. On the other, these results could be affected by surgeon’s practical skills and characteristics of the conduit. Moreover, in-training surgeons could reduce the length of LC with the assistance of expert surgeons or virtual simulations. For that reason, a prospective study including more centers and surgeons, or in alternative, surgeons with different degrees of training could be detrimental.
The present study identifies a three-phases learning curve for pVA creation. Although this procedure is safe and offers acceptable results even in the initial phase of the curve, 25 cases are necessary to reach complete training and 15 more procedures to achieve expert phase. Hence, pVA creation should be performed by expert surgeons or under their direct supervision in specialised institutions.
Authors do not have any potential conflict of interest. They did not receive any financial support or benefit, and they have not financial interest with regard to the work.
Citation: Franchin M, Tozzi M, Mozzetta G, Cervarolo MC, Nahal E, Carcano G, et al. (2019) Prosthetic Vascular Access Creation: learning curve analysis. Angiol Open Access 7:230
Received: 10-May-2019 Accepted: 19-Jun-2019 Published: 28-Jun-2019 , DOI: 10.35248/2329-9495.19.7.230
Copyright: © 2019 Franchin M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.