
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2012) Volume 2, Issue 6
Background: Malaria is a leading cause of maternal and perinatal morbidity and mortality in sub Saharan Africa. Intermittent Preventive Treatment (IPT) with Sulphadoxine-Pyrimethamine (SP) has been proven efficacious in reducing the burden of malaria in pregnancy. However its use is contraindicated in some individuals and malaria resistance to SP has been reported. Therefore there is a need to seek for effective alternatives. This study sought to compare the effectiveness of proguanil versus SP for malaria chemoprophylaxis in pregnancy. Methodology: This was a randomised controlled trial of women attending antenatal clinic at the University of Port Harcourt Teaching Hospital, Nigeria from January 2010 to September 2010. Three hundred and fifty participants were recruited at booking, randomized into two groups using a table of random numbers and monitored till delivery. One group received daily proguanil while the other received SP for malaria prophylaxis. Blood samples were taken for their haematocrit and malaria parasites at booking and delivery. The results were compared. Data management was with SPSS 15 for Windows® statistical soft ware. A p-value of less than 0.05 was considered statistically significant. Results: The prevalence of maternal malaria parasitaemia in this study was 29.9% at booking and 12.5% at delivery. The prevalence at delivery in women given SP and proguanil was 10.6% and 14.4% respectively. This was not statistically significant (P=0.429). There was no statistical difference in the incidence of preterm delivery (P=0.262), cord blood parasitaemia (P=0.385), low birth weight (P=0.175) and birth asphyxia (P=0.367) between the two study groups. Conclusion: There was no significant difference between intermittent preventive treatment with sulphadoxinepyrimethamine and the use of daily proguanil so larger studies with proguanil are warranted.
Keywords: Malaria; Chemoprophylaxis; Pregnancy; Proguanil; Sulphadoxine-Pyrimethamine (SP)
The World Health Organisation (WHO) estimates annually the global incidence of malaria to be about 300 million cases. Malaria is also estimated to kill between 1.1 to 2.7 million people worldwide each year [1,2]. More than 90% of these deaths are from sub-Saharan Africa [1-3]. Over 50 million women are exposed to the risk of malaria every year. Pregnant women are more susceptible to malaria than non pregnant ones and this susceptibility is greatest in first and second pregnancies. Pregnancy complicated by malaria infection results in substantial maternal, fetal and infant morbidity and mortality, causing 75,000-200,000 infant deaths every year [4,5]. Successful control of malaria in pregnancy might therefore prevent these deaths.
In all malarious areas, infection by any of the plasmodium species during pregnancy is detrimental to the mother and the fetus. The most deleterious effects on the mother are caused by plasmodium falciparum [6]. Millions of pregnant women are exposed to malaria and thousands die every year as a result of direct or indirect consequence [6].
Both plasmodium falciparum and plasmodium vivax can cause adverse pregnancy outcomes, including maternal anaemia and low birth weight due to preterm delivery and intrauterine growth restriction [7]. Malaria causes and worsens anaemia in pregnancy, increased uterine activity, abortions and preterm labour [2]. Its effect on the fetus can lead to abortions, IUGR (Intrauterine growth restriction), LBW (Low Birth Weight), severe birth asphyxia, congenital/neonatal malaria, hypoglycaemia and intrauterine fetal death. Cord blood parasitaemia may be present where placental malaria has been active at the time of delivery [8].
Control of malaria during pregnancy depends on both preventing infection and clearing parasitaemia when it occurs [1]. This is because most women in areas of stable malaria transmission may not experience serious clinical illness. The WHO therefore recommends the use of Insecticide-Treated Bed Nets (ITNs), Intermittent Preventive Treatment In Pregnancy (IPTp) and effective case management and treatment of malaria as interventions to prevent this disease in pregnancy [9,10]. Intermittent preventive treatment with Sulphadoxine-Pyrimethamine (SP-IPT) had proven efficacious in reducing the burden of malaria in pregnancy [11]. It is currently the recommended regimen for prevention of malaria in pregnancy in endemic areas. Sulphadoxine-pyrimethamine has been shown to be the most effective single dose anti-malarial drug for prevention of malaria during pregnancy in areas where the strain of plasmodium remains sensitive to it [12]. Current scientific evidence suggests that at least two doses of IPT are required to achieve optimal benefit in most women.
The effectiveness of this drug is recently being threatened by increasing levels of resistance to SP across Africa [13] and South East Asia [14]. There is therefore need to develop alternative drug regimens for IPT in pregnancy. Proguanil is considered as one of the safest anti-malarials currently available [15-17] and has been used extensively for malaria chemoprophylaxis in pregnant women. Proguanil is well tolerated and effective in all trimesters of pregnancy [16]. However, the daily dosing of proguanil raises the problem of client compliance.
Resistance to sulphadoxine-pyrimethamine is increasing, but evidence-based alternatives for IPTp have not yet been determined. In women with contraindications to SP, proguanil is widely used as an alternative. Proguanil had been shown in earlier studies to be an effective chemoprophylactic drug for malaria during pregnancy when used either singly or in combination with other anti-malarials [16,18,19]. However, there is no study on its effectiveness as an alternative to SP in our environment despite its wide use in our practice. This study therefore sought to compare the effectiveness of these two drugs (SP and Proguanil) when used for malaria chemoprophylaxis in pregnancy.
Study site and ethical issues
This study took place in the Obstetrics and Gynaecology department of the University of Port Harcourt Teaching Hospital in Rivers state from January 2010 to September 2010. The University of Port Harcourt Teaching Hospital is a tertiary health institution located in the outskirts of Port Harcourt. Port Harcourt is a metropolitan city located in the heart of the Niger Delta region of Nigeria. The topography is that of flat plains with a network of rivers, tributaries and creeks which have a high potential for breeding of mosquitoes. Malaria transmission is intense year round with a peak during the rainy season months of March to November and a nadir during the dry season months of December to February.
The University of Port Harcourt Teaching Hospital Institutional Review Committee provided ethical approval for the study. Written informed consent was obtained from each study participant.
Study design
This was a randomized controlled trial of women attending antenatal clinic at the University of Port Harcourt Teaching Hospital.
Determination of sample size
The country based survey showed prevalence of malaria parasitaemia in pregnancy to be 23.7% (≈24%) [20]. The sample size for comparison groups, taking an equal number of cases (n1=n2=n′) in the two sub-samples can be calculated from the formula [21,22].
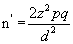
where:
n′=sample size
z=the proportion of normal distribution corresponding to the required significance level (5% ) which is 1.96, which corresponds to 95% confidence level
p=The proportion in the target population estimated to have a particular characteristic. In this case, the proportion of pregnant women with malaria parasitaemia which is 24%
d=differences between two sub-samples, taking an observed difference of 0.10 or more to be significant at the 0.05 level.
The sample size was then calculated as follows:
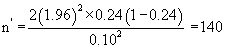
Thus 140 experimental subjects and another 140 control subjects were required for the study. This gave a total of 280 subjects which was rounded up to 300. However, 350 clients were recruited for the study with each arm having 175 subjects. This over sampling was deliberate for drop out and loss to follow up. An average of 170 women booked for antenatal care per week at UPTH. The desired number of women was recruited within six weeks and they were followed up till delivery.
Methods
Three hundred and fifty participants were recruited by random selection at their booking visit after obtaining an informed consent from them. They were then randomized into two groups using a table of random numbers. They picked from concealed numbers which were randomly allocated to contain sulphadoxine-pyrimethamine (Malareich™, Medreich Plc, England) or proguanil (Paludrine™, AstraZeneca UK Ltd). The numbers were taken to the pharmacy where the drugs were stored and dispensed to them. One group was given intermittent preventive treatment for malaria chemoprophylaxis with two doses of SP while the other group received daily proguanil. Two doses of SP were given as DOT (Directly Observed Treatment) after quickening, four weeks apart. This was done under the supervision of nurses at the antenatal clinic and recorded in the patients’ antenatal case notes. Each dose consisted of three tablets of SP containing 500 mg of sulphadoxine and 25 mg of pyrimethamine per tablet (Malareich™). The other group received chemoprophylaxis with 100 mg of proguanil (Paludrine™) daily. The first dose was also given as DOT in order to avoid bias. Those who were taking daily proguanil were told to always take the drug alongside their daily haematinics and bring the packs to the clinic in their next visit. This was done to ascertain drug compliance. Enquiries about side effects were made in the next clinic visit and recorded.
All the participants were given 5 mg of folic acid and 200 mg of ferrous sulphate daily. Participants were properly educated and advised to avoid taking any anti-malarial other than the one given to them. They were told to promptly report to the clinic for proper treatment if they had any malarial symptoms.
Blood was taken at booking from all the participants to check the presence of malaria parasites and haematocrit of the mothers. Maternal and cord blood samples were also taken at delivery to check for hematocrit and malaria parasitaemia. The haematocrit was read using a hand held micro-haematocrit reader while blood film microscopy was used to detect malaria parasitaemia.
The birth weight and Apgar scores were recorded. Demographic information, clinical correlates and outcome measures for each participant were recorded using a designed protocol.
For this study, babies born before 37 weeks of gestation were considered pre-term while those born from 37 weeks of gestation and above were considered term. Low birth weight was defined as neonatal birth weight less than 2,500 g while a haematocrit reading of less than 33% was considered as anaemia.
Exclusion criteria
All women with clinical diagnosis of malaria at booking were excluded from this study. Also excluded were women with multiple pregnancies, HIV positive women, sickle cell disease patients, women with history of allergy to any of the drugs used and patients who had taken anti-malarial drugs prior to booking.
Data analysis
Data collected were recorded into pre-coded case record forms. Thereafter, the data was entered into a spread sheet using SPSS 15 for Windows® (SPSS Inc., Chicago, IL, USA) which was also used for analysis. Descriptive statistics such as means and standard deviations were used to summarize quantitative variables while categorical variables were summarized with proportions. Frequency tables were presented for relevant variables. The student t-test was used to compare two mean values. The Chi-square test was used to investigate associations between two categorical variables and also to compare proportions. For significant associations, the odds ratio (OR) and 95% confidence intervals (CI) were computed. A p-value of less than 0.05 was considered statistically significant.
Three hundred and fifty (350) antenatal clinic attendees were recruited for the study with each arm of the study having 175 subjects. However, only 281 (80.3%) were presented for delivery giving an attrition rate of 19.7%.
Sociodemographic characteristics
Table 1 show that the socio-demographic characteristics of the two groups are similar. The mean maternal age for all participants was 29.6 ± 5.58 years with a range of 16 to 43 years. The median and modal ages were 30 years. The mean age for women who received chemoprophylaxis with Sulphadoxine-Pyrimethamine (SP) was 29.6 ± 5.49 years while that of those who received proguanil was 29.5 ± 5.43 years. A greater proportion of the women aged between 25 to 34 years in groups, 62.7% for SP and 63.3% for proguanil.
| Characteristic | SP n (%) | Proguanil n (%) | P-value |
|---|---|---|---|
| Age Mean age 29.6 ± 5.58 <20 20 – 24 25 – 29 30 – 34 35 – 39 ≥ 40 Total Parity Mean parity 0 1 – 4 ≥ 5 Total Marital status Married Single Total Educational status Primary Secondary Tertiary Total Mean gestational age at booking |
29.6 ± 5.49 years 7 (4.9) 18 (12.7) 40 (28.2) 49 (34.5) 21 (14.8) 7 (4.9) 142 (100) 1.47 ± 1.57 49 (34.5) 86 (60.6) 7 (4.9) 142 (100) 132 (93.0) 10 (7.0) 142 (100) 3 (2.0) 44 (31) 95 (67) 142 (100) 22.01 ± 4.51 |
29.52 ± 5.43 6 (4.3) 22 (15.8) 38 (27.3) 50 (36.0) 16 (11.5) 7 (5.0) 139 (100) 1.42 ± 1.61 53 (38.1) 79 (56.8) 7 (5.0) 139 (100) 135 (97.1) 4 ( 2.9) 139 (100) 5 (3.6) 31 (22.3) 103 (74.1) 139 (100) 17.1 ± 6.85 |
0.954 0.910 0.086 |
Table 1: Sociodemographic characteristics of participants.
The parity of all the clients ranged from 0 to 7 with a mean of 1.44 ± 1.59. The mean parity of women given SP was 1.47 ± 1.58 while that of women who received proguanil was 1.42 ± 1.61. All these parameters were not statistically significant (P>0.05). Most of the clients, 267 (95.0%) were married while 14 (5.0%) were single. Majority of the clients, 198 (70.5%) had tertiary education, 75 (26.7%) had secondary education, while 8 (2.8%) had primary education. The mean gestational age at booking amongst clients who received SP was 22.01 ± 4.51 while that for those given proguanil was 17.1 ± 6.85 (P=0.086).
Prevalence of maternal malaria parasitaemia
The prevalence of maternal malaria parasitaemia in all the participants as shown in Table 2 was 29.9% and 12.5% at booking and delivery respectively. This was significantly lower at delivery (p<0.001).
| Chemoprophyl axis | Booking | Delivery | X2 | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| MP (+) | MP (-) | Total | MP (+) | MP (-) | Total | |||
| SP | 46 (32.4%) | 96 (67.6%) | 142 (100%) | 15 (10.6%) | 127 (89.4%) | 142 (100%) | 0.94 | 0.429 |
| Proguanil | 38 (27.3%) | 101 (72.7%) | 139 (100%) | 20 (14.4%) | 119 (85.6%) | 139 (100%) | ||
| Total | 84 (29.9%) | 197 (70.1%) | 281 (100%) | 35 (12.5%) | 246 (87.5%) | 281 (100%) | 25.60 | < 0.001 |
Table 2: Maternal malaria parasitaemia at booking and delivery in relation to mode of chemoprophyl axis.
The prevalence of maternal malaria parasitaemia at delivery amongst women who had chemoprophylaxis with SP was 10.6% compared to 14.4% among those who received proguanil. This difference was not statistically significant (P=0.429).
The prevalence of malaria at booking was also observed to be more in women who were Para 0 (43.1%) compared to women of higher parities (22.3%). This was statistically significant with a P-value=0.0004.
Clinical malaria occurred in 6.3% of women who received chemoprophylaxis with SP and 5.0% of those who had proguanil. There was no statistical difference. All were cases of uncomplicated malaria and were treated on an out-patient basis with artemeter-lumefantrine combination.
Prevalence of maternal anemia
Maternal anaemia was present in 62.6% of all the clients at booking. There was a significant decrease in the prevalence of maternal anaemia to 19.6% at delivery. This was statistically significant at P<0.0001 (Table 3). Nineteen percent (19.0%) of the women who received chemoprophylaxis with SP were anemic at delivery in comparison with 18.7% of the women who received proguanil. This difference was not statistically significant (P=0.931).
| Chemoprophyl axis | Booking | Delivery | X2 | P-value | ||||
|---|---|---|---|---|---|---|---|---|
| PCV <33% | PCV ≥33% | Total | PCV <33% | PCV ≥33% | Total | |||
| SP | 90 (63.4%) | 52 (36.6%) | 142 (100%) | 27 (19.0%) | 115 (81.0%) | 142 (100%) | 0.01 | 0.931 |
| Proguanil | 86 (61.9%) | 53 (38.1%) | 139 (100%) | 26 (18.7%) | 113 (81.3%) | 139 (100%) | ||
| Total | 176 (62.6%) | 105 (37.4%) | 281 (100%) | 55 (19.6%) | 226 (80.4%) | 281 (100%) | 107.61 | <0.0001 |
Table 3: Maternal anaemia at booking and delivery in relation to chemoprophyl axis.
Mode of delivery
The mean gestational age at delivery in all clients was 38.69 ± 1.72 weeks with a range of 33 to 43 weeks. The mean gestational age at delivery for the group on SP was 38.82 ± 1.66 weeks, while for the group on proguanil it was 38.57 ± 1.78 weeks. This difference was not statistically significant (p=0.853).
Two hundred (71.2%) of the clients had spontaneous vaginal delivery, 74 (26.3%) were delivered by caesarean section while 7 (2.5%) had instrumental vaginal delivery. There was no difference in the mode of delivery for the two groups
Foetal outcome
The foetal outcome is shown on Table 4. Twenty four (8.5%) of all the clients had preterm deliveries with 9 (6.3%) and 15 (10.8%) occurring in women who received SP and proguanil respectively. This difference was not significant (P=0.262). The mean birth weight for all clients was 3306 ± 501 g with a range of 1800 to 4700 g. Women given SP had a mean birth weight of 3303 ± 454 g while those who were given proguanil had a mean birth weight of 3309 ± 547 g (P=0.972). A total of 23 (8.2%) babies had low birth weight. Of these, 8 (5.6%) and 15 (10.8%) were born to mothers who received SP and proguanil respectively. There was no statistical difference between the two groups (p=0.175).
| Parameter | Total | SP | Proguanil | P-value |
|---|---|---|---|---|
| Mean GA at delivery (weeks) Preterm deliveries Mean birth weight ( gram) Low birth weight (gram) Mean Apgar score in the 1st minute Mild Birth asphyxia Moderate Birth asphyxia Mean cord blood haematocrit (%) Cord blood malaria parasitaemia |
38.69 ± 1.72 24 (8.5%) 3306 ± 501 23 (8.2%) 8.00 ± 0.92 18(6.4%) 4 (1.4%) 46.27 ± 5.31 27 (9.6%) |
38.82 ± 1.66 9 (6.3%) 3303 ± 454 8 (5.6%) 8.01 ± 0.92 10 (7.0%) 1 (0.7%) 45.73 ± 5.40 11 (7.7%) |
38.57 ± 1.78 15 (10.8%) 3309 ± 547 15 (10.8%) 7.99 ± 1.07 9 (6.5%) 3 (2.2%) 46.83 ± 5.17 16 (11.5%) |
0.853 0.262 0.972 0.175 0.971 0.935 0.367 0.914 0.385 |
Table 4: Foetal outcome.
All participants had a mean Apgar score of 8.00 ± 0.99 in the 1st minute. The mean Apgar score in the 1st minute for women who received SP was 8.01 ± 0.92 in comparison to those given proguanil with a mean Apgar score of 7.99 ± 1.07 in the 1st minute (P=0.971).
Birth asphyxia was found in 22 (7.8%) of the babies. All the babies had normal Apgar scores in the 5th minute. Mild birth asphyxia occurred in 10 (7.0%) babies and moderate birth asphyxia in 1 (0.7%) of the babies born to mothers who received SP while women given proguanil delivered 9 (6.5%) babies with mild birth asphyxia and 3 (2.2%) with moderate birth asphyxia. This was not statistically significant (P>0.05). None of the mothers delivered a severely asphyxiated baby. There were no stillbirths.
Prevalence of cord blood parasitaemia and anaemia
Cord blood parasitaemia was found in 27 (9.6%) babies and this was higher in nulliparous women (5.3%) than in women of higher parity (4.3%). The difference was statistically significant (P=0.048). The prevalence of cord blood parasitaemia was 7.7% among babies of women who received SP and 11.5% among babies of women given proguanil. There was no statistical difference (P=0.385).
The mean cord blood haematocrit level for all participants was 46.27 ± 5.31% (range 32% to 57%). The mean cord blood haematocrit was 45.73 ± 5.40% for babies born to mothers who received SP and 46.83 ± 5.17% for those who received proguanil. There was no statistical difference between the two groups (p=0.914).
Safety evaluation
Both SP and proguanil were well tolerated in this study. There was no incidence of pruritus, fixed drug eruptions or allergic reactions attributable to any of the chemoprophylactic agents used by any of the mothers. There were no congenital malformations or deaths (maternal or neonatal) among the study participants.
Statistical analysis showed that the sociodemographic characteristics of participants who received chemoprophylaxis with SP and those given proguanil were well matched with no significant difference. The prevalence of maternal asymptomatic malaria parasitaemia in all participants was 29.9% at booking which reduced to 12.5% at delivery and this was significant. The only species of plasmodium incriminated was Plasmodium falciparum. This prevalence (29.9%) was lower than that reported in Lagos (42.2%) [23] and Enugu (58.4%) [24] and could be due to the fact that the study was carried out during the period of minimal rainfall in the area. It was however higher than the country based survey which showed a prevalence of 23.7% [20]. This shows that malaria is still a major problem in our environment.
There was however a significant drop in maternal malaria parasitaemia at delivery following the use of SP and proguanil for chemoprophylaxis during pregnancy. This drop was statistically similar in women who were given SP and those who received proguanil. It had been shown by several authors that IPT-SP is effective in reducing the prevalence of maternal and placental malaria parasitaemia among parturient women [25-29]. It then follows that proguanil may be a good alternative to SP in the reduction of maternal and placental parasitaemia among pregnant women.
Clinical malaria occurred in 5.7% of all participants in this study. The rate of occurrence was similar in the two groups. This figure is similar to the 9.5% clinical malaria rates with the use of SP for malaria prophylaxis recorded in Ibadan South-West Nigeria [25]. All cases were uncomplicated malaria and treated on an outpatient basis. This highlights the importance of routine malaria chemoprophylaxis during pregnancy.
Malaria parasitaemia was significantly more prevalent among primigravidae than in women of higher parity. This is a pointer to the increased susceptibility of this group of women to the disease. This is similar to the finding at Enugu where asymptomatic malaria parasitaemia was more common in the primigravidae [24].
Anemia in pregnancy was found in 62.6% of all clients at booking. This figure dropped significantly to 19.6% at delivery. There was no statistical difference in the prevalence of anaemia at delivery in women who received chemoprophylaxis with SP in comparison to proguanil, indicating that both drugs were equally effective in contributing to the reduction in maternal anaemia. Malaria is a known significant contributor to maternal anaemia though anaemia in pregnancy is multifactorial. Anemia is known to have a negative impact on pregnancy outcome with low birth weight babies and still births occurring more often in anemic mothers [30]. The beneficial effect of these anti-malarials on the reduction of the prevalence of maternal anemia is an important finding which would lead to improved favorable pregnancy outcomes. This finding is in agreement with other reports from West Africa [31].
The foetal outcome in this study was good. The prevalence of low birth weight and prematurity were 8.2% and 8.5% respectively. This is comparable to findings from other studies [25]. Low birth weight and prematurity are the greatest risk factors for neonatal morbidity and major contributors to infant mortality. There was no significant difference in the foetal outcome (preterm delivery, low birth weight) between the two groups showing that both drugs are equally effective. There was no record of stillbirth in either group.
The prevalence of cord blood malaria parasitaemia was 9.6% in all participants. There was no statistical difference between the two groups. This finding is similar to findings of 10.5% from another urban centre in Nigeria [25] but much less than the 24.5% recorded in Mali. This disparity may be because malaria transmission is more intense in rural than urban areas [31]. The Mali study was carried out in semi rural settings while this study was carried out in an urban city. The mean cord blood haematocrit in this study was 46.27% and there was no statistical difference between both groups.
The study reveals the effectiveness of both sulphadoxine-pyrimethamine and proguanil as malaria chemoprophylaxis in pregnancy and there was no significant difference in the efficacy of both drugs in the control of malaria among pregnant women. Proguanil had been shown in earlier studies to be an effective malaria chemoprophylactic drug in pregnancy when used either singly or in combination with other anti-malarials [16,18,19]. It is well tolerated and used in all trimesters of pregnancy.
Intermittent preventive therapy with SP is attractive because of its single dose therapy and its administration supervised in the antenatal clinic, thus ensuring compliance. It is also cheaper and should be used as first choice malaria prophylaxis however, where there are contraindications to the use of SP or in areas where there is resistance to SP, proguanil is an effective alternative.
This study did not take into consideration the use of anti-vector measures such as insecticide treated nets (ITNS) which have been shown to be effective in the prevention of malaria in pregnant women. This is one limitation of this study. However insecticide treated nets are not commonly used. A study in southern Nigeria reported that only 1.1% of the population used ITNs. Therefore this may not affect the outcome of this study.
This small trial did not show a significant difference between intermittent preventive treatment with Sulphadoxine-Pyrimethamine and the use of daily proguanil for malaria chemoprophylaxis in pregnant women. Hence larger studies with proguanil are warranted.
More research on the safety and efficacy of anti-malarial drugs or drug combinations in pregnancy is therefore recommended in order to have a wide range of alternatives where resistance to some of the safe and effective drugs used in pregnancy exists. The use of such drugs in combination with Long Lasting Insecticidal Nets (LLINs) and other vector control measures will enhance the prevention of this disease during pregnancy. The ongoing research into development of safe malaria vaccines should also be intensified as this will help in curtailing the scourge of this disease. However, total eradication of malaria in our environment must be our ultimate goal.
We would like to express our gratitude to the mothers and neonates who participated in this study. We thank the authorities of the University of Port Harcourt Teaching Hospital for granting us the permission to conduct this study. We also thank the Head and all staff of the department of Obstetrics and Gynaecology especially the staff of the antenatal clinic, labour ward and neonatal unit in which this study was conducted for their support and assistance through the period of the study. Our special gratitude goes to our research staff.