
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2020)
Background: Current practice guidelines for patients with Pulmonary Arterial Hypertension (PAH) recommend a multidimensional risk assessment, but World Health Organization (WHO) Functional Class (FC) remains a main criterion for treatment decisions.
Objectives: A post hoc analysis was conducted to determine if different risk assessment tools evaluated in PAH registry populations can identify patients in WHO FC II at higher risk of death.
Methods: Patients in the randomized, controlled EARLY trial (NCT00091715; N=185), which exclusively enrolled patients in FC II, were stratified into three categories—low-, intermediate-, and high-risk—using the original REVEAL risk calculator, revised REVEAL risk calculator 2.0, COMPERA categorical score, and two FPHN methods (invasive and non-invasive) counting low-risk criteria. Risk of all-cause death was compared between baseline categories. Associations between change in risk category from baseline to month 6 (improved, worsened, or stable) and PAH worsening or death were estimated using a Cox proportional hazards model, adjusting for baseline risk category.
Results: Patients classified as intermediate or high risk ranged from 35% using the original REVEAL risk calculator to 89% by the COMPERA method at baseline, and from 37% to 82%, respectively, at month 6. Higher risk category was associated with increased mortality risk. Rates of subsequent PAH worsening and death were higher in patients with worsened risk category and lower in those with improved risk category.
Conclusion: Multiparametric assessment has additional prognostic value over FC alone, but different risk assessment tools vary in risk stratification. PAH patients in WHO FC II are not all at low risk, so should be assessed frequently.
Prognosis; Pulmonary arterial hypertension; Risk assessment; Risk prediction; Survival
Clinical worsening in Pulmonary Arterial Hypertension (PAH) leads to increasingly debilitating symptoms, high morbidity, frequent hospitalizations, and ultimately, right heart failure and premature death [1-4]. Current treatment guidelines for PAH stress the importance of considering patients’ risk for clinical worsening and death. The 2018 update of the CHEST Guideline and Expert Panel Report on therapy for PAH in adults recommends that choice of drug therapy incorporates a methodical evaluation of disease severity and the risk for further short-term deterioration [5]. The 2015 European Society of Cardiology (ESC) and European Respiratory Society (ERS) Guidelines for the diagnosis and treatment of pulmonary hypertension introduced a riskstratification framework to determine the management strategy for each patient with PAH. The framework categorises patients as being at low, intermediate, or high risk for clinical worsening or death, based on clinical, functional, exercise, non-invasive, and invasive variables [6]. This approach was supported in the 2018 Proceedings of the 6th World Symposium on Pulmonary Hypertension [7].
Several tools have been developed to quantify patients’ risk for death and tested retrospectively using data from PAH registries. Algorithms incorporating different subsets of the variables included in the ESC/ERS risk-stratification framework have been evaluated based on European data from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) [8], the French Pulmonary Hypertension Network (FPHN) registry [9], and the Swedish PAH Registry (SPAHR) [10]. All three of these methods use the risk-category thresholds for each included variable proposed in the ESC/ERS Guidelines. The COMPERA and SPAHR assessments grade each available variable, and then calculate an average grade to assign a patient’s overall risk category [8,10]. The FPHN method is a simple count of the number of variables falling into the low-risk category as defined by the ESC/ERS Guidelines [9].
Whereas the COMPERA, FPHN, and SPAHR risk assessments consider only clinical measures, the prognostic equation developed a decade ago to identify predictors of survival in the US Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) also includes PAH etiology, age, and sex [11]. Subsequently, the REVEAL risk equation was prospectively validated in newly diagnosed patients, and used as the basis for a calculator that incorporates parameter weights to yield a single risk score for a given patient [12]. A revised version, REVEAL risk calculator 2.0 (REVEAL 2.0), has recently been published, adding history of hospitalization within the last 6 months and estimated glomerular filtration rate, as well as updates to parameter weights or cut-off values for several included variables [13].
Despite the availability of these multidimensional risk assessments and the recognition in treatment guidelines of the importance of considering several variables, World Health Organization (WHO) Functional Class (FC) alone is still a main criterion for treatment decisions. All currently marketed PAH-specific agents include FC in their indication and usage statements [14,15], and the 2018 CHEST Guideline recommendations are based primarily on WHO FC [5]. The 2015 ESC/ERS Guidelines established the overall treatment goal of achieving a low-risk status, and according to the Guidelines, “Specifically, this means bringing and/or keeping the patient in WHO FC II whenever possible” [6]. However, since many patients in WHO FC II continue to experience disease progression and premature death, all patients in FC II should not be regarded as being at low risk [16,17]. Disease progression may be preceded by changes in right-ventricular structure and function even in patients with stable FC [18]. Consequently, it would be of considerable value to clinicians to be able to identify subsets of patients in WHO FC II at elevated risk.
The EARLY trial (NCT00091715) remains, to date, the only Randomized Controlled Trial (RCT) to enrol exclusively patients with PAH in WHO FC II [19]. Data from EARLY provide the opportunity to evaluate the ability of different risk assessment tools to identify patients at higher risk even when all are in FC II at baseline. The objectives of the present post hoc analyses were to apply different risk assessment methods to data from EARLY to define risk categories in patients with PAH in FC II, and to compare outcomes across risk categories. Risk assessment was performed using the original REVEAL risk calculator and REVEAL 2.0, as well as the COMPERA and FPHN algorithms. The SPAHR assessment was not performed because it differs from the COMPERA method only by including echocardiographic variables, which were not collected in EARLY.
Patients and treatments
Complete inclusion and exclusion criteria for the international, multicenter, double-blind, randomized, placebo-controlled EARLY study have been reported previously [19]. Briefly, eligible patients had PAH in WHO FC II, were aged ≥ 12 years, and had a 6-minute walk distance (6MWD) <80% of the normal predicted value [20] or <500 m associated with a Borg dyspnea index of ≥ 2. Patients were randomly assigned to receive bosentan (n=93) or placebo (n=92) for the 6-month double-blind treatment period. After doubleblind treatment, patients were allowed to receive active treatment through an Open-Label Extension (OLE) study [21]. Irrespective of OLE enrolment, all patients were followed for 5 years. The present analyses included the full trial population of 185 patients.
Ethics: The EARLY trial was conducted in accordance with the amended Declaration of Helsinki. As previously reported, local institutional review boards or independent ethics committees approved the protocol, and written informed consent was obtained from all patients [19]. The present post hoc analysis did not involve any additional interventions on human or animal subjects.
Early endpoints
The pre-specified efficacy and safety endpoints of EARLY and their results were reported by Galiè et al. [19], and will not be repeated in the present article.
Analysis
Risk assessment: For these analyses, patients were pooled regardless of their treatment assignment and stratified into three risk categories: low-, intermediate-, and high-risk. The stratification was performed separately using five different methods (see Table 1 for details): (i) the original REVEAL risk calculator [12], using a prognostic equation derived by a Cox proportional hazard model fit to data from REVEAL [11]; (ii) REVEAL 2.0, an updated risk calculator based on the original REVEAL risk calculator [13]; (iii) the risk stratification categorical score evaluated using data from COMPERA [8]; and two different versions of the count of low-risk criteria evaluated using data from the FPHN registry [9], namely (iv) an invasive method including hemodynamic measures from right heart catheterization and (v) a non-invasive modification substituting N-terminal pro-brain natriuretic peptide (NT-proBNP) for hemodynamic measures. Risk assessments were performed based on available variables. Missing values were counted as 0 risk score in REVEAL risk assessments or excluded from counts of low-risk variables in FPHN risk assessments. For REVEAL 2.0, no hospitalization in the last 6 months (0 risk score) was assumed for all patients at baseline since hospitalization information was not collected at baseline in EARLY.
For each risk stratification method, the proportion of low-, intermediate-, and high-risk patients was summarized for baseline and month 6. Changes in risk category from baseline to month 6 were categorized as improved, worsened, or stable—improved was defined as a decrease in risk category by at least one level, worsened as an increase in risk category by at least one level, and stable as remaining in same risk category as at baseline.
Outcomes: The risk of all-cause death was estimated using the Kaplan–Meier method and compared between baseline risks categories using a Cox proportional hazards model. Hazard Ratios (HRs) for comparisons were calculated with the low-risk category used as the reference.
A landmark analysis of risk of PAH worsening or death by change in risk category was performed following methods of Dafni [22]. PAH worsening was defined as in the EARLY OLE: all-cause death, initiation of intravenous or subcutaneous prostanoids, atrial septostomy, and lung transplantation [21]. The association between change in risk category at month 6 and PAH worsening or death was estimated using a Cox proportional hazards model, adjusting for baseline risk category. The landmark analysis started from month 6 and included all randomized patients who were alive (or without previous PAH worsening) and not lung transplanted at that time-point, and who had non-missing risk score values at both baseline and month 6. For all of these comparisons, HRs were calculated with the “stable” category used as the reference.
Categorical variables are summarized as n (%), and continuous variables are summarized as mean (standard deviation). Event rates and HRs are reported with their associated 95% confidence intervals. Tests of statistical significance were not performed for these post hoc analyses. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Patient characteristics
Full details of characteristics of the patients at baseline in EARLY were previously reported [19], and are presented in Supplementary Table 1. Briefly, all 185 patients had WHO FC II PAH; 69.7% were female, 90.8% were white, and their median age was 42 years. PAH etiology was 60.5% idiopathic or familial, 17.3% congenital heart disease, 17.8% connective tissue disease, 3.8% HIV, and 0.5% other.
| Original REVEAL risk score calculator [12] | REVEAL 2.0 [13] | COMPERA risk stratification [8] | FPHN invasive risk assessment [9] | FPHN non-invasive risk assessment [9] | |
|---|---|---|---|---|---|
| WHO Group 1 subgroup (PAH etiology) | CTD-PAH: +1 | CTD-PAH: +1 | – | – | – |
| PoPH: +2 | PoPH: +3 | ||||
| FPAHa: +2 | FPAHa: +2 | ||||
| Demographics and comorbidities | Renal insufficiencyb: +1 | eGFR <60 mL/min/1.73 m2 or renal insufficiency if eGFR is unavailablec: +1 | – | – | – |
| Males aged >60 y: +2 | Males aged >60 y: +2 | ||||
| WHO/NYHA FC | I: -2 | I: -1 | I/II: 1 | I/II: low risk | I/II: low risk |
| III: +1 | III: +1 | III: 2 | |||
| IV: +2 | IV: +2 | IV: 3 | |||
| Hospitalization | – | All-cause hospitalizations ≤6 mo: +1 | – | – | – |
| Vital signs | SBP <110 mmHg: +1 | SBP <110 mmHg: +1 | – | – | |
| Heart rate >92 bpm: +1 | Heart rate >92 bpm: +1 | ||||
| 6MWD, m | ≥ 440: -1 | ≥ 440: -2 | >440: 1 | >440: low risk | >440: low risk |
| – | 320–<440: -1 | 165–440: 2 | |||
| ≤ 164: +1 | <165: +1 | <165: 3 | |||
| BNP/NT-proBNP, ng/L | BNP <50 or NT-proBNP <300: -2 | BNP <50 or NT-proBNP <300: -2 | BNP <50 or NT-proBNP <300: 1 | – | BNP <50 or NT-proBNP <300: low risk |
| – | BNP 200–<800: +1 | BNP 50–300 or NT-proBNP 300–1400: 2 | |||
| BNP >180 or NT-proBNP >1500: +1 | BNP ≥ 800 or NT-proBNP ≥ 1100: +2 | BNP >300 or NT-proBNP >1400: 3 | |||
| Echocardiogram | Pericardial effusiond: +1 | Pericardial effusiond: +1 | – | – | – |
| DLCO, % pred.e | ≥ 80: -1 | – | – | – | – |
| ≤ 32: +1 | <40: +1 | ||||
| RAP, mmHg | >20 within 1 y: +1 | >20 within 1 y: +1 | <8: 1 | <8: low risk | – |
| 8–14: 2 | |||||
| >14: 3 | |||||
| PVR, Wood units | >32 (2560 dyn·s/cm5): +2 | <5: -1 | – | – | – |
| Cardiac index, L/m/m2 | – | – | ≥ 2.5: 1 | ≥ 2.5: low risk | – |
| 2.0–2.4: 2 | |||||
| <2.0: 3 | |||||
| SvO2, % | – | – | >65: 1 | – | – |
| 60–65: 2 | |||||
| <60: 3 | |||||
| Risk stratification in present study | Sum of scores | Sum of scores | Average of scores for non-missing variables, rounded to the next integer | Count of number of low-risk criteria | Count of number of low-risk criteria |
| Low | 1–7 | ≤ 6 | 1 | ≥ 3 | 3 |
| Intermediate | 8–9 | 7–8 | 2 | 2 | 2 |
| High | ≥ 10 | ≥ 9 | 3 | ≤ 1 | ≤ 1 |
aData collection in EARLY had a combined etiology category of “Idiopathic or Familial (Primary)” PAH rather than separate categories for idiopathic and familial PAH; all patients in this etiology category were scored for FPAH.
bIdentified from adverse event coded with one of the following Preferred Terms: "RENAL FAILURE", "RENAL IMPAIRMENT" and "RENAL DISORDER".
ceGFR was reported under renal insufficiency; if eGFR was reported, then eGFR was used first, otherwise “renal insufficiency” was used if eGFR was unavailable.
dIdentified from adverse event coded with the Preferred Term “PERICARDIAL EFFUSION”.
ePulmonary function testing was not performed in the EARLY study and therefore DLCO does not contribute to the risk score calculation in the present analyses.
Abbreviations: BNP: Brain Natriuretic Peptide; bpm: Beats Per Minute; COMPERA: Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension; CTD-PAH: Pulmonary Arterial Hypertension Associated With Connective Tissue Disease; DLCO: Diffusing Capacity Of Lung For Carbon Monoxide; eGFR: Estimated Glomerular Filtration Rate; FC: Functional Class; FPAH: Familial Pulmonary Arterial Hypertension; FPHN: French Pulmonary Hypertension Network; NT-proBNP: N-Terminal Pro-Brain Natriuretic Peptide; NYHA: New York Heart Association; PAH: Pulmonary Arterial Hypertension; PoPH: Portopulmonary Hypertension; pred.: Predicted; PVR: Pulmonary Vascular Resistance; RAP: (mean) Right Atrial Pressure; REVEAL: Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management; SBP: Systolic Blood Pressure; SvO2: Mixed Venous Oxygen Saturation; WHO: World Health Organization.
Table 1: Risk assessment methods.
Risk stratification
Risk assessment using the original REVEAL, REVEAL 2.0, COMPERA, and FPHN invasive and non-invasive methods categorized a substantial proportion of patients as intermediate or high risk at baseline and month 6 (Figure 1). However, the distribution of patients by risk category varied considerably among risk assessment tools. Across the five methods, the proportion of patients classified as being at intermediate or high risk ranged from 35% to 89% at baseline, and from 37% to 82% at month 6. The original REVEAL method yielded the most low-risk patients; the COMPERA method yielded the least.
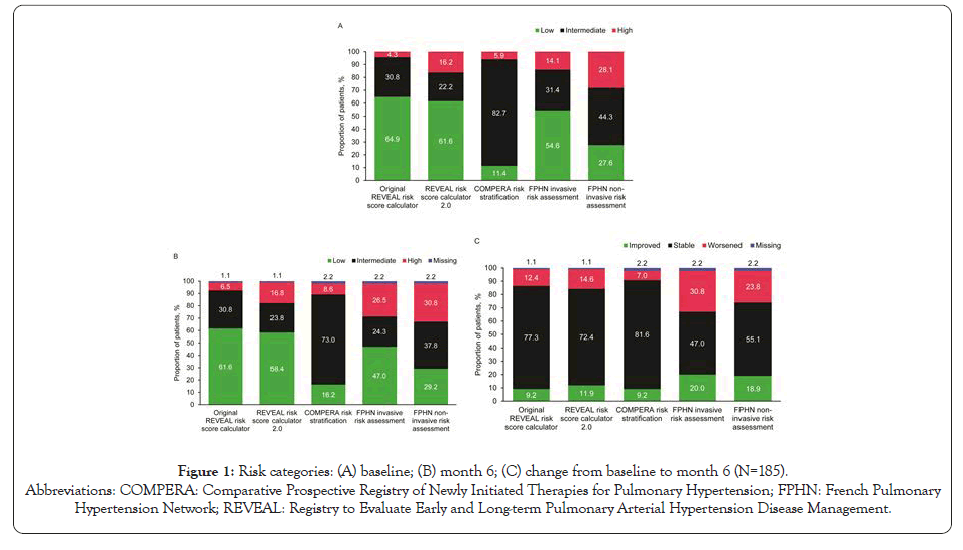
Figure 1: Risk categories: (A) baseline; (B) month 6; (C) change from baseline to month 6 (N=185).
Abbreviations: COMPERA: Comparative Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension; FPHN: French Pulmonary
Hypertension Network; REVEAL: Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management.
Component variables responsible for classifying patients as being at intermediate or high risk despite all being in WHO FC II at baseline are reported in Supplementary Table 2–5. For the original REVEAL method and REVEAL 2.0, PAH etiology was the variable with the highest proportion of patients (79%) scored for elevated risk. For the COMPERA and FPHN methods, the only variable with more than 50% of patients not in the low-risk category at baseline was 6MWD. A substantial percentage of patients also had BNP or NT-proBNP levels scored for elevated risk, but approximately 15% of patients had missing data for these variables.
| Risk assessment method | Change from baselinea | Hazard ratiob (95% confidence interval) | |
|---|---|---|---|
| PAH worsening | All-cause mortality | ||
| Original REVEAL risk score calculator | Improved | 0.34 (0.10–1.14) | 0.27 (0.06–1.19) |
| Worsened | 1.11 (0.43–2.87) | 1.36 (0.46–3.99) | |
| REVEAL risk score calculator 2.0 | Improved | 0.48 (0.18–1.30) | 0.23 (0.05–0.99) |
| Worsened | 2.73 (1.28–5.82) | 2.24 (0.91–5.51) | |
| COMPERA risk stratification | Improved | 0.36 (0.09–1.52) | 0.22 (0.03–1.67) |
| Worsened | 2.60 (0.97–6.97) | 2.24 (0.66–7.63) | |
| FPHN invasive risk assessment | Improved | 0.84 (0.38–1.86) | 1.23 (0.50–3.04) |
| Worsened | 1.08 (0.54–2.17) | 1.39 (0.60–3.25) | |
| FPHN non-invasive risk assessment | Improved | 0.46 (0.20–1.08) | 0.48 (0.18–1.31) |
| Worsened | 1.00 (0.48–2.06) | 1.16 (0.51–2.67) | |
aReference group: Stable.
bCox proportional hazards model adjusted for baseline risk category.
Abbreviations: COMPERA: Comparative Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension; FPHN: French Pulmonary Hypertension Network; PAH: Pulmonary Arterial Hypertension; REVEAL: Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management.
Table 2: Relationships between change in risk category (baseline to month 6) and PAH worsening and death.
Impact of risk category on outcomes
For all of the risk assessment tools, patients in the intermediate- and high-risk categories at baseline had numerically higher mortality rates and increased HRs (>1.0) compared with those in the low- risk category (Figure 2), indicating that higher risk category was associated with increased mortality risk.
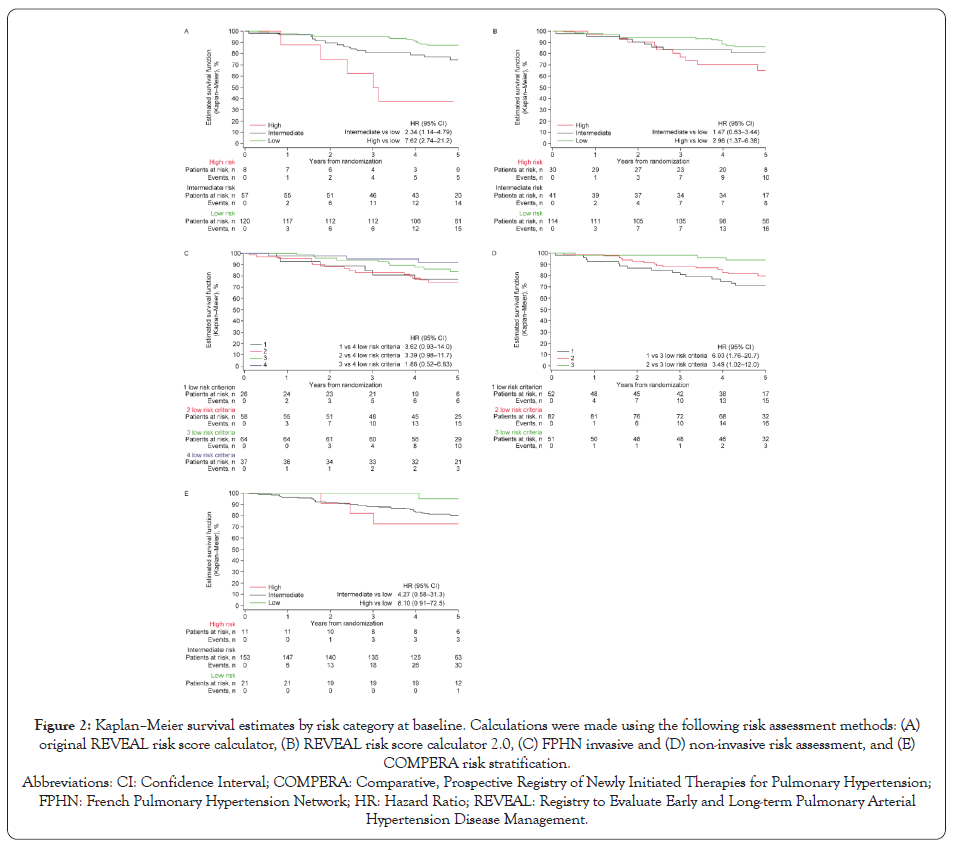
Figure 2: Kaplan–Meier survival estimates by risk category at baseline.
Calculations were made using the following risk assessment methods: (A)
original REVEAL risk score calculator, (B) REVEAL risk score calculator 2.0, (C) FPHN invasive and (D) non-invasive risk assessment, and (E)
COMPERA risk stratification.
Change in risk category
Worsening of risk category from baseline to month 6 occurred in 7% to 31% of patients, depending on risk assessment tool, with the COMPERA method indicating the least worsening and FPHN invasive method the most (Figure 1).The proportion of patients with improved risk category was lower for the original REVEAL and COMPERA methods (both 9%) and REVEAL 2.0 (12%) than for the FPHN invasive and non-invasive methods (20% and 19%, respectively).
Change in risk category and outcomes
The landmark analyses revealed that, compared with “stable” patients who remained in the same risk category from baseline to month 6, those with worsened risk category over this period experienced numerically higher rates of subsequent events of PAH worsening and death, and those with improved risk category had numerically lower rates of these events (Table 2).
These findings demonstrate that many patients in WHO FC II are at elevated risk for death, and that patients at increased risk can be identified on the basis of measurable clinical characteristics. However, risk stratification and the apparent strength of the association between risk category and subsequent mortality differed substantially among the five assessment methods evaluated. While the widest separation in mortality between high- and low-risk categories was seen with the original REVEAL risk calculator stratification, we are unable to conclude that this method is superior to the others we evaluated, because our post hoc analyses did not formally compare the statistical significance of associations between risk categories and outcomes among the different risk assessment tools.
Our results support more widespread use of risk assessment tools in the management of patients with PAH traditionally considered being at low risk. Despite the call in the 2015 ESC/ ERS Guidelines for regular multidimensional risk assessment [6], a 2017 survey of 94 cardiologists and pulmonologists in France, Germany, Italy, and the USA revealed infrequent use of several guideline-recommended prognostic measures in follow-up of their PAH patients [23]. Furthermore, 80% of individual patients judged by physicians in this survey to be at low risk were classified at higher risk according to the COMPERA method, highlighting discordance between physicians’ opinions of patients’ risk status and objective risk-category calculations.
A strength of the present analyses is that they are based on data from a prospective RCT. The Proceedings of the 6th World Symposium on Pulmonary Hypertension highlighted limitations in testing risk assessment algorithms against observational registries that do not all have standardized data collection, and have substantial missing data and loss to follow-up [7]. Our results extend the findings of Frost et al., who applied the original REVEAL risk calculator to patients in the AMBITION RCT of first-line ambrisentan plus tadalafil combination therapy [24]. In that analysis, only 37% of patients in WHO FC II were classified as low risk (REVEAL risk score <6), while 53% were at intermediate risk (score 6–8) and 10% were at high risk (score >8). The authors did not report outcomes by risk category separately for patients in FC II, but noted that their findings support the view that patients newly diagnosed with PAH in FC II are not necessarily at low risk.
Humbert et al. (2019) applied the FPHN non-invasive and COMPERA/SPAHR methods to data from PATENT-1/-2 RCTs of riociguat in patients with PAH [25]. Patients achieving ≥ 1 lowrisk criteria or a low-risk category at follow-up had a significantly reduced risk of death and clinical worsening, compared with patients achieving no low-risk criteria or an intermediate-risk category. Results were not reported separately for patients in FC II.
Using the original REVEAL risk calculator [12], Benza et al. found that change in risk score from enrollment in REVEAL to 12 months significantly predicted 1-year survival [26]. Most of the mortality HRs we obtained were in the expected direction (i.e.,>1 for patients who worsened and <1 for patients who improved). Although the 95% confidence intervals of this HRs were wide and most crossed 1.0, this could partly reflect the relatively small numbers of patients who changed risk category.
Although risk groups were classified based on probability of death, our analysis suggested that change in risk category was also associated with subsequent PAH worsening in WHO FC II patients. The risk assessment tools were mainly developed to assess risk of death rather than clinical worsening, but the development of REVEAL 2.0 included an evaluation of risk of clinical worsening [13]. Frost et al. and Humbert et al. also demonstrated that risk assessment tools have prognostic value for clinical worsening [24,25].
The distribution of patients by risk category in EARLY differed considerably with different assessment algorithms. All available risk assessment tools have inherent limitations, and none perfectly captures an individual patient’s risk [27, 28]. Further studies are needed in order to determine which variables are most predictive of outcome, or if variables are interchangeable [29]. Recently, additional biomarkers (high sensitive troponin-T, high sensitive C-reactive protein, galectin-3, red blood cell distribution width) were found to be associated with event-free survival in PAH, but they did not provide additional prognostic value independent of the REVEAL risk score [30].
An important limitation of the present study is that these are post hoc analyses in which the PAH worsening events were prespecified as part of the secondary endpoints. Also, PAH worsening events in EARLY were assessed by the study investigators [19] and were not independently adjudicated as in recent event-driven PAH clinical trials (i.e., SERAPHIN [31], AMBITION [32], GRIPHON [33], and FREEDOM-EV [34]).
Our main finding was that not all patients with PAH in WHO FC II from the EARLY study are at low risk, and risk assessment applied to this cohort correlates with observed clinical outcome. The multiparameter assessment tools vary in separation of risk profiles even when applied to the identical population. Patients traditionally felt to be low risk due to WHO FC II status should receive risk assessment with appropriate close monitoring to promptly identify any need for treatment optimization, potentially including escalation of therapies.
Medical writing and editorial support were provided by W. Mark Roberts, PhD, Montreal, QC, Canada, funded by Actelion Pharmaceuticals US, Inc., a Janssen Pharmaceutical Company of Johnson & Johnson.
Drs Kim, Lickert, Pruett, and Ms Zhao participated in study design and data analysis. Drs Lickert and Pruett and Ms Zhao acquired the data. All authors contributed to study conception, data interpretation, manuscript drafting and/or critical revision, approved the final manuscript, and agree to be held accountable for all aspects of the work.
Dr Kim has received grant support from Bellerophon, Eiger, Gilead, Lung Biotechnology, and SoniVie; personal fees for consultancy, steering committee work, and speaker bureau membership from Actelion Pharmaceuticals US, Inc., a Janssen Pharmaceutical Company of Johnson & Johnson and Bayer; and personal fees for consultancy from Arena, Merck, and SteadyMed.Drs Lickert, Pruett and Drake were employees of Actelion Pharmaceuticals US, Inc., a Janssen Pharmaceutical Company of Johnson & Johnson, when this research was conducted, and hold stock in Johnson & Johnson. Ms Zhao is an employee of Actelion Pharmaceuticals US, Inc., a Janssen Pharmaceutical Company of Johnson & Johnson, and holds stock in Johnson & Johnson.
Actelion Pharmaceuticals Ltd., a Janssen Pharmaceutical Company of Johnson & Johnson, was the sponsor of the Efficacy and Safety of Oral Bosentan in Pulmonary Arterial Hypertension Class II (EARLY) trial. Actelion Pharmaceuticals US, Inc., a Janssen Pharmaceutical Company of Johnson & Johnson provided the design and statistical analysis plan for the data analyses described in this article, conducted the data analyses, and participated in data interpretation, preparation of the manuscript, and the decision to publish the manuscript.
Citation: Kim NH, Lickert CA, Pruett JA, Zhao C, Drake W (2020) Prognostic Value of Risk Assessment Tools for Patients with Pulmonary Arterial Hypertension in WHO Functional Class II: A Post Hoc Analysis of the Early Trial. J Clin Trials. 10:443.
Received: 25-Nov-2020 Accepted: 08-Dec-2020 Published: 15-Dec-2020
Copyright: © 2020 Kim NH, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.