Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Research Article - (2019)Volume 7, Issue 2
Introduction: Central Nervous System involvement (CNSi) in patients with Acute Myeloid Leukemia (AML) rarely occurs. It is not well characterized clinically and lacks standardized treatments.
Patients and methods: A retrospective analysis of 77 consecutive AML patients with primary and secondary CNSi during 2004-2016 was performed in eight Polish haematological centres.
Results: 77 patients (38 with primary CNSi-AML) were included. Median age was 44 years in both groups. Elevated lactate dehydrogenase activity was found in the majority of subjects. Patients in primary CNSi-AML group more often had myelomonocytic and monoblastic AML subtypes (68.4%) compared to secondary CNSi AML (43.5%) (p=0.039). There were no differences between both groups in the number of leukocytes or the proportion of blast cells rates in peripheral blood or bone marrow at AML diagnosis. There were also no statistically significant differences between both groups in the incidence of cytogenetic or molecular abnormalities. In both groups, CNSi was most frequently found in the cerebrospinal fluid and the most common neurological symptom was headache. The following manifestations were more frequent in secondary than in primary CNSi-AML: lower extremity weakness (38.46% vs. 13.16%; p=0.023), paraesthesia (38.46% vs. 13.16%; p=0.023), motor deficits (31.58% vs. 10.53%; p=0.047), and asymmetry of reflexes (26.32% vs. 2.7%; p=0.007). Median pleocytosis was also significantly higher in secondary than in primary CNSi-AML: 27 (IQR 2-146) vs. 2 (IQR: 1-12; p=0.004). Both groups had rather short Overall Survival (OS), with a median of 16.6 months (9.9-NA) for patients with primary CNSi-AML and 15.4 months (10.1-21.1) for patients with secondary CNSi.
Conclusion: Patients with CNSi AML were relatively young, having high lactate dehydrogenase activity and high rates of the myelomonocytic and monoblastic/monocytic AML subtypes. The advisability of undertaking CNS examination and prophylaxis in patients with such characteristics thus merits further reassessment.
Central Nervous System involvement (CNSi); Acute Myeloid Leukemia (AML)
The origins of Acute Myeloid Leukemia (AML) lie in precursor tumour-transformed hematopoietic cells, which lead to clonal proliferation and the accumulation of morphologically and functionally immature blast cells [1-5]. AML rarely invades areas other than the bone marrow, but in such instances, the most commonly afflicted organs are soft tissues, lymphatic tissues, and skin [6-9]. Central Nervous System involvement (CNSi) is rare [10-13].
In recent years, there has been an ever-growing interest in CNSi during the course of AML (CNSi-AML). Diagnosis rates of CNSi-AML have risen due to the increasing use of flow cytometry to analyze Cerebro-Spinal Fluid (CSF). This technique is more sensitive than cytological examination and has thus enabled more cases of early-stage CNSi-AML to be identified [14,15]. CNSi during leukemia can occur simultaneously with bone marrow involvement, or it may precede myeloid localization; these situations constitute primary involvement of the CNS [16]. Secondary involvement of the CNS, in contrast, is the appearance of leukemia cells at this location in the event of relapse or resistance to treatment.
Because the CNS is so rarely involved, neither routine diagnostics nor prophylactic measures are undertaken in AML patients [3,17] and the literature is thus confined to reporting small adult patient groups [10-13,18-27]. The incidence rate of CNSi-AML at AML diagnosis is estimated at 0.6%-5%, whilst the range at recurrence is 3%-15% [10-14,28]. Risk factors for CNSi include elevated Lactate Dehydrogenase (LDH) activities, hiperleucocytosis (leucocyte numbers>50 G/L), myelomonocytic/monoblastic/monocytic subtypes, presence of inv (16) mutation, coexistence of Fms-related tyrosine kinase 3 (FLT3-IDT) mutation with a Nucleophosmin 1 (NPM1) mutation, myeloid/lymphoid or Mixed-Lineage Leukemia (MLL) gene rearrangements, a complex karyotype, blast cell CD56 expression and age below 2 years [11,13,22,23,29-36].
CNSi-AML has a poor prognosis [10,11,13,24,35]. Response rates to treatment are satisfactory, but there are high rates of recurrence nevertheless [34]. Furthermore, in cases of CNSi- AML and for other sites outside the bone marrow, a weak Graftvs.- Leukemia (GvL) effect is observed after allo-HSCT [28,37]. No standards have been established for the types of systemic and intrathecal chemotherapy to use in CNSi-AML patients [17,38]. Because CNSi-AML is so infrequent, precise clinical characterizations of such patients are lacking, as are standardized treatments. Moreover, it remains unclear whether the clinical presentation and risk factors differ for cases of primary CNSi or during the disease course. This study therefore presents the clinical features and treatment course of CNSi-AML patients treated in haematological centres in Poland in 2004-2016, taking into account the division between those with primary and secondary CNSi.
A retrospective analysis of medical record data of CNSi-AML patients treated in selected Polish hematological tertiary care referral centres was performed. The eligibility criteria were as follows:
• age ≥ 18 years,
• AML diagnosis during 2004-2016 and
• CNSi at any stage of the disease. The study was conducted in accordance with the Declaration of Helsinki.
Defining CNSi
CNSi was defined as CSF involvement, cranial nerve involvement with signs of paralysis or myeloid sarcoma infiltration of the brain and/or spinal cord. Cerebrospinal fluid samples were examined by conventional cytology and with 6-8 color flow cytometry. CSF mononuclear cells were distinguished from debris by their forward/sideward light scatter. Lymphocytes and monocytes were identified by their light scatter properties, CD45 pattern and their CD3 and CD14/CD64 expression, respectively. The presence of tumor cells in CSF was confirmed by detecting population expressing CD117, CD34, CD13 and CD33.
Involvement of the CSF was determined as being overt with a pleocytosis of at least 5 cells/μL in the presence of leukemic cells in the smear sediment and/or upon immunophenotypic testing of the CSF. Occult CSF involvement was recognised when pleocytosis was less than 5 cells/μL in the presence of leukemic cells in sediment cytology and/or in immunophenotypic testing of the CSF.
Myeloid sarcomas identified by imaging tests (Computed Tomography (CT), and/or Nuclear Magnetic Resonance (NMR)) of the brain and/or spinal cord required histopathology to confirm the aetiology of AML.
The group of patients with primary CNSi included those in whom CNSi was found at AML diagnosis (as being either the only site or one coexisting with bone marrow involvement and/or any other organ). The group of patients with secondary involvement included those who had CNSi at further stages of AML treatment, as in AML relapses or in the course of AML that was initially resistant to chemotherapy. Isolated AML in the CNS was defined as CNSi without involvement of the bone marrow or any other organs within the ensuing 30 days. Lumbar punctures were performed when neurological symptoms occurred and less often when the high risk factors for CNSi were present.
Imaging tests (CT and/or NMR) were performed when the patient had neurological symptoms and whenever overt or occult CSF involvement was found.
Clinical and laboratory parameters
The following clinical features were recorded: age, gender, blood morphology, LDH activity levels, percentage of blasts in bone marrow, CD56, CD2 and CD7 antigen expression on tumour cells following flow cytometry of the bone marrow, cytogenetic abnormalities determined by G-banding or, in certain cases, by FISH (Fluorescence In-Situ Hybridisation) and molecular abnormalities determined by the Polymerase Chain Reaction (PCR). AML subtypes were also assessed according to the 2016 WHO classification (World Health Organization Classification) as well as by the former FAB (French-American-British) Classification together with the cytogenetic risk grouping according to the SWOG (Southwest Oncology Group) [7,39,40].
Treatment responses
All available therapeutic data were analyzed. CNS remission was defined as the absence of leukemia cells in sediment smears of the CSF and/or upon CSF testing using flow cytometry, combined with a lack of CNS infiltration as determined by imaging analysis.
The duration of Overall Survival (OS) was defined as the interval from the time when AML was diagnosed to the death of the patient from any cause or to the last observation. The duration of Disease-Free Survival (DFS) was defined as the interval from the time when CNSi-AML was diagnosed until relapse, death during remission or the last observation.
Statistical methods
Summary statistics were used to describe the variables of interest, i.e., the mean, standard deviation, median and Inter Quartile Range (IQR) or the number and percentage/ proportion, depending on the variables’ distribution. Groups were compared by the chi-squared test or Fisher's exact test for categorical variables, whilst Student’s t-test or Wilcoxon test was used for continuous invariables. Survival curves were constructed using the Kaplan-Meier estimator and compared using the log-rank test.
All tests used were two-tailed, and a significance level of p<0.05 level was taken to indicate a significant difference. The statistical software used was the R Package version 3.0.1 from the R Development Core Team, in Vienna, Austria.
This retrospective study included 77 consecutive patients suffering from CNSi-AML, of whom 49 (63.64%) were males. The overall median age was 44 (IQR 28-57) years. Primary CNSi was observed in 38 cases, whilst secondary CNSi was observed in 39.
Features of the subject group with primary CNSi
Patient’s characteristics: There were 38 such subjects, of whom 26 (68.42%) were males. The median age was 44 years (IQR; 31-55). In six patients (15.8%), isolated involvement of the CNS was found, whilst the remaining 84.2% also had bone marrow or extra medullary involvement. A significant finding was that in 33 (89%) cases, elevated levels of LDH activities were measured. The patients’ characteristics are shown in Table 1.
| AML diagnosis | Primary CNSi AML N=38 (49.35%) | Secondary CNSi AML N=39 (50.64%) | |
|---|---|---|---|
| Gender, N (%) | p=0.532 | ||
| Female | 12 (31.58) | 16 (41.03) | |
| Male | 26 (68.42) | 23 (58.97) | |
| Age, median (IQR) | 44 (31-55) | 44 (28-57) | p=0.783 |
| WBC, median x 109/L (IQR) | 38.7 (10.5-102,4) | 46 (6.3-139.6) | p=0.843 |
| Platelet count, median x 109/L (IQR) | 58 (32.5-92) | 42 (24-88) | p=0.695 |
| Blood blasts or their equivalents, median (%), (IQR) | 55 (40-78) | 46.5 (17-80) | p=0.589 |
| Medullar blasts or their equivalents, median (%), (IQR) | 66 (49.5-77.5) | 76 (58-90) | p=0.064 |
| Elevated lactate dehydrogenase, Lactate dehydrogenase in normal range, median number of patients, N (%) | 33 (89.19), 4 (10.81) | 35 (94.59), 2 (5.41) | p=0.674 |
| Bone marrow | |||
| CD 56 expression, median (IQR) | 33 (2.5-68) | 5.7 (0.2-79.2) | p=0.315 |
| CD 7 expression, median (IQR) | 46 (0.9-25) | 1 (0.2-14) | p=0.192 |
| CD 2 expression, median (IQR) | 15 (0-14) | 0.8 (0.1-8.7) | p=0.851 |
CNSi, central nervous system involvement; AML, acute myeloid leukaemia; IQR, Interquartile range; WBC, white blood cell count; LDH, lactate dehydrogenase
Table 1: Characteristics of patients with primary and secondary CNSi-AML (N=77).
The myelomonocytic subtype was found in fifteen (41.67%) patients, the monoblastic type in eleven (30.56%), and characteristics of AML with maturation in eight (22.22%).
Karyotypes were determined in 36/38 subjects (94.73%), and metaphase assessment was possible in 30 (78.94%). Cytogenetic abnormalities such as 3, 8, 11, 16, 21 chromosome abnormalities, del(5q), monosomy 7, t(9;22), t(15;17), complex and monosomal karyotype were assessed.
Normal karyotypes were found in eight (26.66%) of the patients who had had their karyotypes evaluated. Cytogenetic abnormalities in chromosome 8 were found in eleven patients (36.66%), of whom three (10%) had the t(8;21) abnormality. Abnormalities in chromosome 11 were observed in three cases (10%) and in chromosome 16 in eight cases (26.66%), of whom six (20%) had the inv(16) abnormality. A 5q deletion was seen in one case (3.33%), monosomy 7 in two cases (6.66%), a t(9;22) abnormality also in 2 cases (6.66%), a t(15;17) in one case (3.33%), abnormalities in chromosome 21 in three cases (10%), abnormalities in chromosome 3 in two instances, and a complex or monosomal karyotype in five patients (16.66%). Other cytogenetic abnormalities were found only in a few patients.
With regard to molecular prognostic factors, analyses of the t(8;21)(q22;q22.1) transcript of RUNX1-RUNX1T1 were performed in 28 (73.68%) patients, in whom four such cases were detected (14.28%), whereas CBFB-MYH11 analyses were performed on 22 (57.8%) cases of inv(16), with mutations seen in three (13.63%); meanwhile, there were seven (21.87%) mutations observed upon analysing FLT3-ITD in 32 cases (84.21%).
However, for other molecular factors such as PML-RARA alpha, overexpression of the BAALC gene, NPM1, MLL-PTD, c-kit, BCR/ABL, JAK2 V617F and MLL-AF4, data were available from only a few patients. Because the FLT3-ITD mutant allele fraction had not been measured, and due to the small number of subjects tested for NPM1 mutation, any assessment of the cytogenetic and molecular at-risk groups was thus impossible according to ELN 2017.
Table 2 shows the breakdown of patients according to the WHO 2016 classification of AML, cytogenetic risk according to the SWOG classification and the previous FAB classification.
| Primary CNSi-AML N=38 (49.35%) | Secondary CNSi-AML N=39 (50.64%) | ||
|---|---|---|---|
| WHO 2016 acute myeloid leukaemia classification, N (%) | p=0.964 | ||
| AML with recurrent genetic abnormalities | 10 (26.3) | 9 (23.7) | |
| AML with myelodysplasia-related changes | 3 (7.9) | 4 (10.5) | |
| Therapy related myeloid neoplasms | 0 (0) | 1 (2.6) | |
| AML, NOS | 23 (60.5) | 21 (55.3) | |
| Myeloid sarcoma | 2 (5.3) | 2 (5.3) | |
| Acute leukaemias of ambiquous lineage | 0 | 1 (2.6) | |
| SWOG classification, N (%) | p=0.962 | ||
| Favourable | 5 (17.86) | 5 (16.67) | |
| Intermediate | 8 (28.57) | 10 (33.33) | |
| Unfavourable | 8 (28.57) | 9 (30) | |
| Not known | 9 | 9 | |
| Not done | 7 (25) | 6 (20) | |
| AML subtypes according to the previous FAB classification, N (%) | p=0.144 | ||
| AML with minimal differentiation | 0 (0) | 0 (0) | |
| AML without maturation | 1 (2.78) | 6 (17.4) | |
| AML with maturation | 8 (22.22) | 9 (25.71) | |
| APL | 1 (2.78) | 3 (8.57) | |
| Acute myelomonocytic leukaemia | 15 (41.67) | 8 (22.86) | |
| Acute monoblastic/monocytic leukaemia | 11 (30.56) | 9 (25.71) | |
| Not determined | 2 | 4 | p=0.039 |
| Other than myelomonocytic/monoblastic/ monocytic leukaemia | 12 (31.6) | 22 (56.4) | |
| Myelomonocytic/monoblastic/ monocytic leukaemia | 26 (68.4) | 17 (43.6) |
CNSi: Central Nervous System involvement; AML: Acute Myeloid Leukaemia; WHO: World Health Organisation; NOS: Not Otherwise Specified; SWOG, Southwest Oncology Group; FAB, French-American-British; APL: Acute Promyelocytic Leukaemia
Table 2: WHO 2016, SWOG and previous FAB classification of CNSi AML patients (N=77).
Features of CNSi: CNSi-AML was predominantly diagnosed as CSF involvement in 35 subjects (91.2%). Cranial nerve involvement was observed in two (5.3%) patients, whereas four (10.5%) demonstrated spinal cord involvement and another four (10.5%) showed intra-cerebral infiltration. Mixed forms with involvement of CSF and the cranial nerves were found in two (5.3%) cases, CSF involvement and intra-cerebral infiltration in four (10.5%), and CSF and spinal cord infiltration in one (2.6%).
The most commonly found neurological symptoms were headaches (17 patients; 44.74%) and alterations of mental state (eight patients; 21.05%).
Cerebrospinal fluid examination, including an evaluation of immunophenotype by flow cytometry, was undertaken in 35 (92.1%) patients.
A general examination of CSF found a median pleocytosis (cell number/μL) of 2 (IQR: 1-12), a median percentage of leukemic cells in the CSF sediment smear of 29 (IQR: 0-79) and a median percentage of leukemic cells of 39.1 (IQR: 15.5-66) as determined by immunophenotyping.
The detailed CNSi characteristics are included in Table 3.
| Primary CNSi-AML N=38 (49.35%) | Secondary CNSi-AML N=39 (50.64%) | ||
|---|---|---|---|
| CNSi localization, N (%) | |||
| CSF/meningitis leukemia | 35 (92.1) | 34 (87.2) | p=0,711 |
| Intracranial myeloid sarcoma | 4 (10.5) | 7 (18.0) | p=0.517 |
| Spinal cord myeloid sarcoma | 4 (10.5) | 8 (20.5) | p=0,517 |
| Neurological signs/symptoms, N (%) | |||
| Headache | 17 (44.74) | 16 (41.03) | p=0.921 |
| Nausea/vomiting | 5 (13.16) | 6 (15.38) | p=1 |
| Cranial nerve palsy | 3 (7.8) | 10 (25.64) | p=0.141 |
| Seizure | 2 (5.26) | 6 (15.38) | p=0.263 |
| Altered mental status | 8 (21.05) | 10 (25.64) | p=0.837 |
| Motor deficits | 4 (10.53) | 12 (31.58) | p=0.047 |
| Increased intracranial pressure | 3 (7.89) | 4 (10.26) | p=1 |
| Diffuse back pain | 5 (13.16) | 9 (23.08) | p=0.405 |
| Lower leg weakness | 5 (13.16) | 15 (38.46) | p=0.023 |
| Paresthesias/ dysaesthesia | 5 (13.16) | 11 (28.21) | p=0.178 |
| Sphincter dysfunction | 3 (7.89) | 7 (17.95) | p=0.31 |
| Asymmetry of reflexes | 1 (2.7) | 10 (26.32) | p=0.007 |
| Nuchal rigidity | 4 (10.53) | 4 (10.25) | p=1 |
| Meningeal signs | 3 (7.89) | 2 (5.26) | p=1 |
| CSF examination | |||
| Leukocyte count/µL- median (IQR) | 2 (1-12) | 27 (2-146) | p=0.004 |
| Protein level (mg/dL)-median (IQR) | 38 (27-62) | 51 (29.13-77) | p=0.272 |
| Glucose level (mg/dL)- median (IQR) | 59.54 (52.78-65) | 60 (50-76.1) | p=0.370 |
| Erythrocyte count/µL - median (IQR) | 0 (0-32.5) | 0 (0-1) | p=0.181 |
| Cell differential (% of leukemic cells) - median (IQR) | 29 (0-79) | 70 (6-93.5) | p=0.077 |
| CSF flow cytometry (% of leukemic cells) – median (IQR) | 39.1 (15.5-66) | 66.8 (6-96) | p=0.093 |
| AML localization, N (%) | |||
| Isolated CNSi | 6 (15.8) | 12 (30.76) | p=0.001 |
| CNSi and BMi | 30 (78.9) | 17 (47.22) | |
| CNSi, BMi and other EM sites | 2 (5.3) | 3 (7.7) | |
| CNSI and other EM sites involvement | 0 (0) | 7 (17.9) |
CNSi: Central Nervous System involvement; AML: Acute Myeloid Leukaemia; CSF: Cerebro-Spinal Fluid; IQR: Inter Quartile Range; BMi: Bone Marrow involvement; EM: Extramedullar
Table 3: Central nervous system involvement characteristics (N=77).
Treatment: Systemic chemotherapy had been given to 35 (92.1%) patients (most often the 3+7 regimen). Cladribine was additionally given to three (9.6%) patients. Patient with Acute Promyelocytic Leukemia (APL) was treated according to the PETHEMA regimen.
Intrathecal chemotherapy was given to 37 (97.37%) patients using cytarabine and methotrexate. Radiotherapy was administered to eight (21%) patients, where ‘Total-Body Irradiation’ (TBI) was given to two patients (31.57%) as part of their conditioning prior to allogeneic Hematopoietic Stem Cell Transplantation (allo-HSCT).
Remission in the CNS after systemic and intrathecal treatment was found in 23 patients (67.65%). Allo-HSCT at CR1 (first Complete Remission) was performed in 12 patients (49.3%), whilst allo-HSCT at CR>1 was used in five patients.
The likelihood of DFS after one year was 52.63%, (95% CI: 35.81-66.95), whilst the likelihood of OS after one year was 57.89% (40.75-71.69), with the median OS being 16.6 months (90-NA).
The likelihood of achieving OS after one year in those who had undergone allo-HSCT at CR1 was 100% (95% CI: NA-NA), compared to allo-HSCT at CR>1, which was 40.0% (95% CI: 5.2-75.3), p=0.001.
The median observation times were 20.7 (16.3-39.6) and 11.3 (IQR: 10.3-16.6) months, respectively.
The probability of OS after one year in the group of patients who did not undergo allo-HSCT was 40.6% (95% CI: 19.6-60.7) with a median OS follow-up of 5.1 months (IQR: 1.1-13.3). Details of treatment and outcomes as well as the likelihood of DFS and OS are presented in Table 4.
| Primary CNSi-AML (n=38; 49.35%) | Secondary CNSi-AML (n=39; 50.64%) | ||
|---|---|---|---|
| Intrathecal chemotherapy, N (%) | 37 (97.37) | 34 (87.18) | p=0.2 |
| Systemic chemotherapy after CNSi diagnosis N (%) | 36 (94.74) | 33 (86.84) | p=0,43 |
| With cladribine | 5 (14.29) | 8 (25) | p=0.007 |
| With etoposide | 1 (2.86) | 6 (18.75) | |
| With fludarabine | 1 (2.86) | 5 (15.62) | |
| With clofarabine | 1 (2.86) | 0 (0) | |
| Radiotherapy, N (%) | 10 (26.3) | 5 (12.8) | p=0.244 |
| Brain radiotherapy | 8 (21) | 5 (12.8) | |
| TBI before allo-HSCT | 2 (5.2) | 0 (0) | |
| Outcomes in CNS, N (%) | |||
| Remission | 23 (67.65) | 12 (33.33) | p=0.03 |
| Non remission | 9 (26.47) | 17 (47.2) | |
| Recurrent disease | 2 (5.88) | 5 (13.89) | |
| Death before assessment | 0 | 2 (5.56) | |
| Lack of the data | 4 | 3 | |
| DFS | |||
| Relapse, N (%) | 13 (34,2) | 30 (76.92) | p=0.047 |
| Relapse or death, N (%) | 25 (65,8) | 37 (94.9) | |
| 1-year DFS probability [95%CI] | 52,63 (35.81-66.95) | 37.28 (22.33-52.24) | |
| Median DFS (years) | 1,08 (0,43-2.58) | 0.88 (0.68-1.16) | |
| OS | |||
| Death, N (%) | 23 (60.53) | 31 (79.49) | p=0.561 |
| 1-year survival probability [95%CI] | 57.89 [42.3-73.4] | 60.88 [45.2-76.0] | |
| Median OS (months) | 16.6 (9.9-NA) | 15.4 (10.1-21.1) | |
| Survival probability from the time of CNSi diagnosis | |||
| Death, N (%) | 23 (60.53) | 31 (79.49) | p=0.004 |
| 1-year survival probability (95%CI) | 57.89 (42.3-73.4) | 26.8 (13.5-41.9) | |
| Median OS (months) | 16.6 (9.9-NA) | 4.4 (1.4-7.1) |
CNSi: Central Nervous System involvement; AML: Acute Myeloid Leukaemia; allo-HSCT: allogeneic Hematopoietic Stem Cell Transplantation; DFS: Disease-Free Survival; OS; Overall Survival; IQR: Inter-Quartile Range; CI: Confidence Interval
Table 4: Treatment and outcomes of patients with primary and secondary CNSi-AML (N=77).
Features of the subject group with secondary CNSi Patient’s characteristics: The subjects consisted of 39 patients with secondary CNSi-AML, of whom 23 (58.97%) were males. The median age was 44 (IQR 28-57) years. In 24 (61.5%) patients, CNSi-AML was diagnosed at AML recurrence (first or subsequent) after chemotherapy, whilst the diagnosis was made in seven (17.9%) patients at AML relapse (first or subsequent) after allo-HSCT, in seven (17.9%) in AML patients who were initially refractory to chemotherapy and in one patient with remission in the bone marrow but without neurological symptoms where CNSi-AML had been diagnosed by routine CSF testing prior to allo-HSCT.
CNSi was found after a median of eight months (IQR 3-15 months) from when AML was diagnosed. In twelve patients (30.76%), the CNS alone was involved; seventeen (47.22%) had CNS and bone marrow involvement; in three (7.7%) cases there were involvements of the CNS, bone marrow and other locations; and in seven (17.9%) there was involvement of the CNS and at other locations outside the bone marrow.
Similar to the other patient group with primary CNSi-AML, this patient group had a notably high rate of elevated LDH activity, i.e., in 94.59% of cases. Nine (25.71%) patients showed characteristics of AML with maturation, while the monoblastic subtype was found in nine (25.71%) and the myelomonocytic subtype was found in eight (22.80%). The patients’ characteristics are shown in Table 1.
Karyotyping was performed in 37 (94.87%) patients, with metaphase assessment being possible in 33 (89.18%) cases, and normal karyotypes were found in 11 (33.33%) instances. Cytogenetic abnormalities such as 3, 8, 11, 16, 21 chromosome abnormalities, del(5q), monosomy 7, t(9;22), t(15;17), complex and monosomal karyotype were assessed.
Cytogenetic abnormalities in chromosome 8 were found in eight (24.24%) patients, including two (6.06%) who possessed the t(8;21) abnormality. Chromosome 11 abnormalities were demonstrated in three (9%) cases, and chromosome 16 abnormalities in four (12.12%); there were no cases of del(5q) or monosomy 7; meanwhile, the t(6;9;22) abnormality was found in one (3.03%) patient, the t(15;17) in two (6.06%) patients, chromosome 21 abnormalities in one (3.03%), chromosome 3 abnormalities in one (3.03%) and a complex or monosomal karyotype in six (18.18%) patients. Other cytogenetic abnormalities were found only in a few patients.
Molecular testing for the RUNX1-RUNX1T1 t(8;21) transcript (q22;q22.1) was performed in nineteen (48.71%) patients, with the transcript detected in three (15.78%); for the CBFB-MYH11 inv(16) in seventeen cases (43.58%), with mutations demonstrated in four (23.52%); and for FLT3-ITD mutation in 27 (69.23%) cases, of which seven were found to have this mutation (25.92%).
All the remaining molecular factors (PML-RARA alpha, overexpression of the BAALC gene, NPM1, MLL-PTD, c-kit, BCR/ABL, JAK2 V617F and MLL-4) were tested in only a few instances. It was not possible to analyse the cytogeneticmolecular risk groups according to ELN 2017 because the FLT3- ITD mutant allele fraction had not been measured and only few subjects were tested for NPM1 mutation.
The breakdown of patients according to the WHO 2016 classification of AML, and cytogenetic risk according to the SWOG classification as well as previous FAB classification are presented in Table 2.
Features of CNSi: The most common site of CNSi was the CSF (35 subjects; 91.2%). Spinal cord involvement was found in three cases, while one had cranial nerve involvement and one showed intra-cerebral infiltration. Mixed forms with CSF involvement, intracerebral infiltration or cranial nerve and spinal cord involvement were observed in fifteen (38.46%) patients. Two cases (5.12%) had only CSF involvement and intracerebral infiltration.
Headaches were the most common neurological symptom, occurring in sixteen patients (41.03%), whereas paraesthesias occurred in fifteen patients (38.46%) and weakness of the lower limbs was found in fifteen patients (38.46%).
A general cytological investigation of the CSF, with immunophenotyping, was undertaken in 35 (89.7%) patients. This investigation showed a median pleocytosis (cell count/μL) of 27 (IQR: 2-146), a median percentage of leukemic cells in the CSF sediment smear of 70 (IQR: 6-93.5), and a median percentage of leukemic cells by immunophenotyping of 66.8% (IQR: 6-96). The detailed CNSi characteristics are shown in Table 3.
Treatment: Before being diagnosed with CNSi, all patients in this group received radical chemotherapy. Most patients (89.74%) received the 3+7 regimen, where cladribine was additionally given to six (17.14%) patients, whilst one (2.85%) received fludarabine.
In patients with acute promyelocytic leukemia, idarubicin and cytarabine and ATRA was used in two cases (5.71%), and idarubicin and ATRA was given in one (2.85%). Twelve patients (30.76%) received allo-HSCT in CR1.
After CNSi was diagnosed, systemic chemotherapy based on the 3+7 regimen was used on 36 patients (94.74%). Of these patients, eight were additionally treated with cladribine, six with etoposide and five with fludarabine.
Intrathecal chemotherapy was administered to 34 (87.18%) patients, where the vast majority received cytarabine and methotrexate, whilst in two isolated cases a liposomal form of cytarabine was given. Radiotherapy of CNS was performed in 5 (12.8%) patients in the whole group. In 11 (28.2%) patients achieving CR allo- HSCT was performed (for 8(20.51%) patients it was second transplant).
The likelihood of achieving DFS after one year was 37.28% (95% CI 22.33-52.24), whilst the likelihood of OS stood at 60.88% (95% CI 43.67-74.28). The median OS from when AML was diagnosed was 15.4 (10.1-21.1) months, whilst the median OS from when CNSi was diagnosed was 4.4 (1.4-7.1) months.
The data are shown in Table 3. The likelihood of achieving OS after one year in patients who had undergone allo-HSCT was 87.5% (38.7-98.1).
Details of treatment and outcomes as well as the likelihood of DFS and OS are presented in Table 4.
The survival curve for patients with CNSi AML is shown in Figure 1.
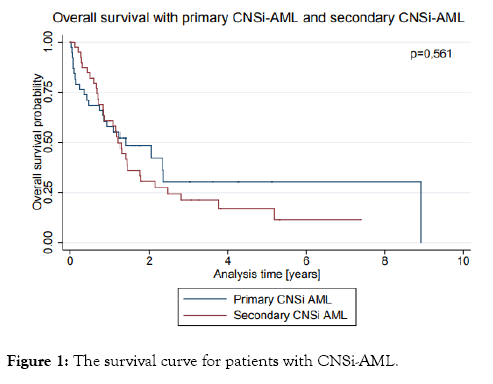
Figure 1: The survival curve for patients with CNSi-AML.
Comparing the primary and secondary CNSi-AML patient groups
Both patient groups had a median age of 44 years, with males slightly predominating (p=0.78 and 0.53 respectively). There were no differences between groups in the number of leukocytes or the proportion of blast cells rates in peripheral blood or bone marrow (p=0.84, 0.58 and 0.064, respectively). Elevated LDH activity was found in the majority of subjects from both the primary and secondary groups (89.19% vs. 94.59%, respectively; p=0.06). Significantly elevated incidences of the myelomonocytic and monoblastic subtypes were observed in those with CNSi at diagnosis (26 patients, 68.4%) compared to patients found to have CNSi during further stages of treatment (17 patients, 43.5%); p=0.039.
There were no statistically significant differences between patients with primary and secondary CNSi in the incidence of cytogenetic or molecular abnormalities (p>0.05 for each variable). The rates at which patients were divided into individual subtypes; i.e., the AML WHO 2016 classification and the cytogenetic risk groups according to the SWOG, did not differ significantly between groups (p=0.96). Simultaneous involvement of the bone marrow and the CNS was more frequent in patients with primary CNSi than in the other study group (i.e. 83.33% vs. 47.22%), p=0.001. In contrast, CNSi with simultaneous involvement of organs other than the bone marrow was more often found in the secondary than the primary CNSi AML group (16.67% vs. 52.77; p=0.001).
In both the primary and secondary CNSi AML groups, leukemia in the CNS were most commonly found in the CSF (92.1% vs. 87.2%; p=0.711). In subjects with secondary CNSi, locations other than the CSF, such as the midbrain, cranial nerves, spinal cord and combinations of those sites, were more common than in primary CNSi (41% vs. 21%; p=0.162). Clinically, headache was the most common symptom in both the primary and secondary groups (44.74% vs. 41.03%; p=0.92). Furthermore, the secondary CNSi group showed higher rates of weakness of the lower limbs (38.46 vs. 13.16%; p=0.023), paraesthesia (38.46 vs. 13.16%; p=0.023), motor deficits (31.58 vs. 10.53%; p=0.047) and asymmetrical reflexes (26.32 vs. 2.7%; p=0.007) than primary CNSi group Significantly higher pleocytosis was observed in the CSF of patients with secondary than primary CNSi AML, with median of 27 (IQR 2-146) and 2 (IQR: 1-12), respectively (p=0.004). No statistically significant differences were found for any of the remaining CSF parameters.
In both groups, short OS times were observed, with a median of 16.7 months (9.0-28.2) months for patients with primary CNSi- AML and 15.4 (10.1-21.1) months for patients with secondary CNSi-AML (p=0.56). The median survival time from the diagnosis of secondary CNSi over the clinical course of AML was 4.4 (1.4-7.1) months.
The first-ever reports of CNSi during the course of AML were published in the 1960s and 1970s [18,41]. Most evidence has been reported from retrospective studies on small groups of subjects. Although 50 years has since elapsed, issues such as incidence, risk factors, recommended treatment and treatment outcomes are still not clear. Another unknown is whether the risk factors and clinical characteristics of CNSi found at the time of AML diagnosis are the same as when CNSi develops at some later time in the course of disease.
This study analysed patient characteristics and treatment course in AML patients with CNSi at diagnosis and at further stages of disease. The median age of our study patient subjects was relatively young (44 years), indicating a predisposition of younger patients to CNSi, particularly if one compares this age to the median age of 67 years upon diagnosis of AML in general [42,43]. Similar ages have been reported elsewhere for CNSi- AML cases [44,45]. Also a multivariate analysis by Cheng et al. demonstrated that an age of 45 years or younger is an independent risk factor for CNSi-AML [11].
Our study showed that patients with secondary CNSi-AML were diagnosed with these CNSi 8 months after leukemia was diagnosed. Other studies have reported similarly short median times to the diagnosis of secondary involvement in AML patients [11,34,44,45]. Such early recurrences of leukemia may indicate that there was a reservoir of blasts already present in the CNS at diagnosis that had not been completely eradicated by standard chemotherapy. It may, therefore, be the case that adding chemotherapeutics that penetrate the CNS during induction therapy, such as cladribine and high dose of cytarabine (during remission consolidation therapy), would better prevent any recurrences in this area, especially in patients at high risk of CNSi.
According to reports in the literature, the risk factors for the primary involvement of the CNS by myeloid leukemia cells are as follows: elevated white blood cell counts when AML is diagnosed; elevated rates of bone marrow blasts; presence of inv(16), MLL gene rearrangements; complex karyotypes; FLT3- ITD mutations; FLT3-ITD mutations coexisting with NPM1 mutations; elevated LDH activity; and myelomonocytic, monocytic or monoblastic proliferation [10,11].
In the presented analysis, the median number of leukocytes and the numbers of blasts found in the peripheral blood and bone marrow were at levels in keeping with medians typically found in AML populations. There were no statistically significant differences between primary and secondary CNSi-AML in the number of white blood cells or in the number of leukemic cells in the peripheral blood and bone marrow. In contrast, we have demonstrated that LDH activity was elevated in the majority of our subjects (89.19% and 94.59%) in both groups. In our study, the myelomonocytic or monoblastic subtypes were more frequently observed in patients with primary CNSi than in those with secondary CNSi (68.4% vs. 43.5%, p=0.039). Nonetheless, it should be emphasised that the myelomonocytic or monoblastic subtype was the most common subtype of leukemia in both groups.
Cytogenetic or molecular risk factors for CNSi include presence of inv(16) mutation, chromosome 8,11 abnormalities, Fmsrelated tyrosine kinase 3 (FLT3-IDT) mutation, coexistence of FLT3-IDT mutation with a Nucleophosmin 1 (NPM1) mutation, myeloid/lymphoid or Mixed-Lineage Leukemia (MLL) gene rearrangements, and a complex karyotype [11,13,22,23,29-36]. There were no statistically significant differences between patients with primary and secondary CNSi in the incidence of cytogenetic or molecular abnormalities in our study. In the literature there is a lack of a similar comparison data.
In both of our study groups, CNSi most often occurred in the CSF. Patients with secondary CNSi were more likely than those with primary CNSi to have involvement at intracerebral sites and mixed locations in the cranial nerves. For these reasons, patients with secondary CNSi were more likely than those with primary CNSi to suffer symptoms such as weakness of the lower limbs, paraesthesia, motor deficits, and asymmetrical reflexes. As mentioned previously, headache and altered mental state were the most commonly observed neurological symptoms, showing the need for appropriate diagnostics in those AML patients presenting such a clinical picture.
Our analysis showed that approximately 90% of subjects with primary or secondary CNSi had received first-line systemic chemotherapy based on the 3+7 regimen. Only a small number of patients with primary CNSi-AML had received additional chemotherapeutics that are potentially able to enter the CNS, such as cladribine. Most patients in both groups had received intrathecal chemotherapy using cytarabine and methotrexate after CNSi diagnosis. Radiotherapy of the CNS had been undertaken in only thirteen (16.8%) study subjects. This rate is lower than that reported by other studies [13], although radiotherapy was not demonstrated to extend the OS of patients with CNSi-AML [23,33,46].
CNSi in AML is considered a poor prognostic factor because of short-term remission along with high relapse rates and short survival time [10,34,47]. The median OS in our study for patients with primary CNSi was 16.6 months, which was similar to the 15.4 months found in our group with secondary CNSi. Nevertheless, the median OS of patients in the latter group from the time of CNSi diagnosis was only 4.4 months. Retrospective studies indicate that the allo-HSCT procedure improves the outcomes of CNS and AML treatment in adults [47]. Our analysis found significantly increased OS in patients with primary CNSi-AML who had had allo-HSCT performed during the first remission. The patient group with secondary CNSi also showed benefits in OS for patients who had undergone allo- HSCT at CR1, as well as those who had undergone the procedure later. We stress that our findings should be interpreted with great caution due to the retrospective nature of our analysis and the necessarily small sample sizes inherent to this study.
The early recurrence of leukemia via CNSi may indicate a reservoir of remaining blasts within the CNS, thereby suggesting the insufficient efficacy of any systemic chemotherapy previously administered. Furthermore, the similar clinical features of our two AML patient groups (i.e., those with primary and secondary CNSi) should be noted. Both groups were relatively young, having high LDH activity and high rates of the myelomonocytic and monoblastic AML subtypes. It is thus apparent that AML patients with such characteristics at diagnosis should be reconsidered for CSF testing in first remission with immunophenotypic evaluation, as well as appropriate prophylaxis for CNSi [2].
Patients with CNSi AML were relatively young, having high lactate dehydrogenase activity and high rates of the myelomonocytic and monoblastic/monocytic AML subtypes. The advisability of undertaking CNS examination and prophylaxis in patients with such characteristics thus merits further reassessment.
Citation: Patkowska E, Szczepaniak A, Baranska M, Kazmierczak M, Paluszewska M, Jedrzejczak WW, et al. (2019) Primary and Secondary Central Nervous System Involvement in Acute Myeloid Leukemia . J Leuk 7: 257. doi: 10.24105/2329-6917.7.257
Received: 14-Mar-2019 Accepted: 04-Apr-2019 Published: 11-Apr-2019 , DOI: 10.35248/2329-6917.19.7.257
Copyright: © 2019 Patkowska E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.