
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2020)Volume 11, Issue 7
Exposure to excess Glucocorticoids (GCs) during embryonic development influences offspring physiology and behaviors and induces change in Hypothalamic-Pituitary-Adrenal (HPA) axis genes expression and serotonergic system in mammals. Whether prenatal corticosterone (CORT) exposure induces similar effects in avian species remains unclear. In the present study, we injected low (0.2 μg) and high (1 μg) doses of CORT in ovo before incubation and detected changes in aggressive behavior, Tonic Immobility (TI), HPA axis and 5-hydroxytryptamine (serotonin) (5-HT) system gene expression on post hatch chickens of different ages. High dose of CORT significantly (P<0.05) suppressed growth rate, increased the frequency of aggressive behaviors, which was associated with elevated plasma CORT concentration. Likewise, in ovo injection of CORT significantly (P<0.05) increased Tonic Immobility (TI) duration both in chickens from low and high doses of CORT treatments compared to control. In addition, administration of CORT significantly (P<0.05) up-regulated mRNA expression of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) whereas it down-regulated 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) and mineralocorticoid receptor (MR) mRNA expression in the hypothalamus. No significant differences were seen in Glucocorticoid Receptor (GR) and 20-hydroxysteroid dehydrogenase (20-HSD) mRNA levels upon CORT treatment. Moreover, CORT exposure significantly (P<0.05) increased hypothalamic 5-hydroxytryptamine (serotonin) receptor 1A (5-HTR1A) mRNA expression, but not 5-HT receptor 1B (5-HTR1B). In ovo administration of CORT may programs the aggressive behaviors in the chicken through alterations of HPA axis and 5-HT system.
Behavior; Chicken; Corticosterone; Glucocorticoid metabolic enzymes; Hypothalamus
The phenotype of an individual is not only programmed by genetic factor, however, it’s also via ecological factors that play a fundamental role in determining offspring phenotype [1], physiology [2], and behavior [3]. In birds, maternal influences have aroused much interest after the discovery that bird's eggs contain a variety of maternal derived steroid hormones [4,5]. Corticosterone (CORT), the main plasma Glucocorticoid (GC) in avian species has been confirmed to be transmitted into chicken’s egg [6]. The CORT concentration in eggs has reported to be modified via a variety of factors including physiological status of the bird [7], stressful surroundings environment [8], and housing system [9].
Fetuses or embryos of mammals and avian species are exposed to a substantial amount of maternal GCs either through the placenta in mammals [10] or by yolk deposition in birds [7]. Chronic stress modulates the Hypothalamic-Pituitary-Adrenal (HPA) axis function results in increased exposure to GCs via elevation in baseline of GCs levels and thus causes a decrease in offspring body weight gain and growth [11,12]. Exposure of GCs during the development of embryos is confirmed to have both short- and longterm outcomes [10,13], for example it reduced offspring weight [14] and compromised immune system function [12]. Prenatal exposure of GC modify the HPA axis activity [15,16] and behavior [17]. In addition, In ovo injection of CORT prior to incubation has found to increase flight performance behavior [18], fearfulness behavior [14]. Furthermore, elevation of egg CORT at later stages of embryonic development enhanced the recall of a passive avoidance task [19], and increased the rate of pecking behavior at grains and pebbles [20].
The intracellular concentrations of active GC are synchronized by a number of GC metabolizing enzymes [21]. 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) triggers [22], while 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) inactivates GCs [23-27]. In mammals, 11β-HSD1 gene mRNA is expressed mainly in liver [28], kidney [29] and lung [30,31], whereas, 11HSD2 mostly expressed in kidney [32], colon [33] and placenta [34]. A number of studies revealed that maternal stress during pregnancy alters 11ß-HSDs expression [35,36]. In mammal, chronic restraint stress throughout pregnancy was also found to reduce 11ß-HSDs mRNA gene expression [37]. Substantial exposure of GCs consider a common relationship between the prenatal surrounding environment [38], fetal growth [39] and adult neuroendocrine [40,41] and affective disorders [42]. The Inhibition of 11β-HSD1 was reported to prevent stress effects on hippocampal synaptic plasticity [43] and impairs contextual fear behavior [43].
In mammals, prenatal stressors are found to reprogram the HPA axis activity and the 5-hydrodxytryptamine (serotonin) (5-HT) system function [44]. Previous studies have revealed that the 5-HT system plays a vital role in modulating aggressive behavior [45]. Elevated serotonergic system activity is commonly influence reduction of aggression [46]. Deregulation of both HPA axis and serotonergic systems are associated with mental health [47] generally and with mood disorder in particular [48]. Low concentrations of blood 5-HT are linked with altered physiological status [49], including the HPA axis [50] and aggressive behavior in humans [51]. In humans, childhood stress is found to induce aggression which associated with disrupted HPA axis function [52] and reduced the 5-HT system function in adulthood [53,54]. The frequency of aggressive behaviors increased in the hen of Dekalb XL (DXL) and low group productivity and survivability (LGPS) hen treated with 5-hydroxytryptamine (serotonin) receptor 1A (5-HT1A) antagonist indicating that serotonin plays a major role in aggressive behaviors [55]. In avian species, the majority of studies that investigating the influences of artificial elevations of egg CORT fare found to focus on growth [56] and behavior [57].
The mode of action of GCs in the cells is mediated through glucocorticoid receptors (GR), and mineralocorticoid receptors (MR) [58]. The intracellular availability of active GC is modulated by pre-receptor mechanisms [21] and corticosterone binding globulin (CBG). The 11β-hydroxysteroid dehydrogenase (11β-HSD1) activates, whereas 11β-hydroxysteroid dehydrogenase (11β-HSD2) deactivates GCs in all animal species [23-27]. In addition, in birds, 20-hydroxysteroid dehydrogenase (20-HSD) is an abundantly and ubiquitously expressed enzyme [22], which transforms GCs to the inactive 20-dihydrocorticosterone [59]. GCs are reported to increase aggressive behavior through both genomic [60] and nongenomic mechanisms [61-63]. The action of GCs is controlled at the hypothalamic and pituitary level [64]. One important regulatory mechanism consists of modulation in the expression of the two isoforms of 11β-HSDs which catalyze the inter-conversion of GCs [65]. Several reports have indicated that the hypothalamus has a role in aggression in different species inducing finches [66], sparrows [67] and rats [68]. Yet, the effects of embryonic CORT exposure on hypothalamic glucocrticoid metabolic enzyme gene, 5-HT receptor expression and its association with aggressive behavior in the of chicken is not reported.
In the present study we used a model of in ovo injection of CORT before incubation to test our hypotheses that aggressive behavior and plasma CORT concentration may be influenced by CORT treatment and these changes may be associated with hypothalamic GCs metabolic enzymes gene and 5-HT receptor expression.
Egg incubation and CORT injection
Two hundred and ten fertilized chicken eggs overall mean mass (64.6 ± 0.44 g) were selected from eggs laid by hens one month after onset of lay and randomly divided into three groups (70 in each group). CORT (Sigma-Aldrich, USA) was dissolved in absolute alcohol, rather than the oil that affected embryonic development of chickens in our previous trials, and diluted in PBS to produce doses of 0.2 μg and 1 μg in a volume of 100 μL solution containing a minimal amount of alcohol. The high and low CORT dose was determined based on previous publications [69,70]; taking into consideration the CORT concentration detected in the yolk (3-4 ng/g) and the albumen (0.5 ng/g) [71]. Before incubation, the eggs were injected with PBS (control) and a 0.2 μg (low) or a 1 μg (high) dose of CORT under aseptic conditions. Eggs were injected randomly by advancing a Hamilton syringe into a hole in the middle of the long axis until the yolk membrane was penetrated (approximately 20 mm below the surface). The incubation conditions were set according to our previous publication [72]. Chicks were hatched inside the incubator and were left to dry completely (up to 12 h) before they were removed. The hatchability of the eggs ranged from 70% to 75% and no obvious differences in hatchability or hatching time were observed among three groups. One-day-old chicks were individually weighed, wing banded, and placed into battery cages with continuous fluorescent lighting. The temperature was adjusted to 32°C –35°C during the first week, and reduced approximately 3°C per week until 21°C. Both sexes were transferred to floor pens covered with sawdust litter. The stock density was 20–25 kg/m2. The relative humidity was maintained at 40%–60%, and the lighting, ventilation, as well as the feeding and management procedures complied with the Feeding Management Regulations of Yellow-feathered Chicken (NY/T 1871-2010). The growth performance was started on Day 1 (D1) posthatch and recorded weekly from hatching to 10 weeks of age. On D133, hens started to lay eggs. On D175, blood samples were collected for plasma CORT measurement. Behaviors test were performed on posthatch D196. On D210 tonic immobility tests were performed twice per day for three days using different batches of chickens. We used 6 animals per group, in total 18 animals for parameters except the growth rate. We tried not to use the same batch of 6 birds for different measurements in order to minimize the stress caused by different manipulations. On D245, all chickens were killed by rapid decapitation one of the physical methods, which have been used as an ethical type of euthanasia. The hypothalami were collected, washed with PBS then put in liquid nitrogen and later kept at -80°C for further analysis. The experiment procedures were approved by the Animal Ethics Committee of Nanjing Agricultural University.
Aggressive Behavior test
The behavior tests were performed on D196 as described previously [73]. Briefly, 30 chickens from each group which were unfamiliar to each other from different brooders were placed in an experimental arena (similar in size and structure to their brooders where chickens have been raised) which was established in a room familiar to the animals. The room was visually and acoustically isolated from the aviary. For visual identification, chickens were marked with different colors (red, green, blue) on different locations (head, back and tail). Neither the colors nor their locations affected the behaviors of chickens in the present study. The chicken's behavior was videotaped during a 60 min period. The number of aggressive attacks of each individual was recorded, and aggression was defined as a chicken pecking, grabbing, twisting skin on the head and nape of the other chicken. The observer who recorded and analyzed the aggressive behaviour was not aware of the experimental treatments.
Tonic Immobility (TI) test
TI tests were measured on D210 using different chickens. The TI tests were measured according to the method described previously [74]. Briefly, a chicken was carried individually to another isolated room devoid of other birds. The chicken was placed on its back on the floor and restrained for at least 20 s (with one hand on the sternum and one lightly cupping the head of the bird). The experimenter remained silent and virtually motionless in the room, out of the bird's sight. The TI duration was considered between 10 and 600 s. If the chicken terminated in <10 s, it was captured, and the trial was repeated. If TI was not attained after 3 attempts, a score of 0 s was given. Conversely, if the bird failed to right itself after 10 min, the test was terminated and a maximum score of 600 s was given for tonic immobility duration.
Plasma CORT assay
The birds used for taking blood samples were trained prior to the sampling to get used to human manipulations. Approximately 1 mL of blood was collected from the jugular vein and duplicate plasma samples (2 × 50 μL) were used for the CORT assay. The plasma CORT concentration was measured with a commercial enzyme immunoassay kit (500655, Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturer’s instructions. The calculated detection limit of the assay was 27 pg/mL and all the determinations fell within the range of detection. The intraassay coefficient of variation was 5%. The cross-reactivity of the antibody was 11% with 11-dehydrocorticosterone, 7% with 11-deoxycorticosterone, 0.31% with progesterone, 0.17% with cortisol, 0.06% with aldosterone, 0.03% with testosterone, 0.02% with pregnenolone, 0.01% with 5α-DHT and less than 0.01% with other steroids.
RNA extraction and mRNA quantification with Real-time PCR
Hypothalamus samples were ground with pestle and mortar in liquid N2 and a portion of approximately 100 mg was used for the RNA extraction using the TRIzol total RNA kit (Invitrogen, Biotechnology Co, Ltd, Carlsbad, CA, USA) according to the manufacturer’s instructions, and reverse transcript to cDNA using 0.5 μg/μL (4μL contains 4μg) of RNA with the PrimeScript RT reagent kit according to the manufacturers instruction (Takara). To investigate the effect of the in ovo injection of CORT on the expression of hypothalamic genes, real-time PCR was performed in an Mx3000P (Stratagene, USA) according to published methods [75]. Mock RT and No Template Controls (NTC) were included to monitor the possible contamination of genomic and environmental DNA at the RT and PCR steps. A pooled sample made by mixing equal quantities of the RT products (cDNA) from all the samples was used for optimizing the PCR conditions and tailoring the standard curves for each target gene, and melting curves were performed to insure a single specific PCR product for each gene. The PCR products were sequenced to validate the identity of the amplicons. Primers specific for the 11β-HSD1, 11β-HSD2, 20-HSD, GR, MR, 5-HTR1A and 5-HTR1B (Table 1) were synthesized by Geneary, Shanghai, China. Chicken β-actin was used as a reference gene for normalization purposes. The method of 2−ΔΔCt was used to analyze the real-time PCR data [76].
| Target genes | Gen Bank accession number | PCR products (bp) | Primer sequences |
|---|---|---|---|
| Β-actin | L08165 | 300 | F : 5′- TGCGTGACATCAAGGAGAAG -3′ |
| R : 5′- TGCCAGGGTACATTGTGGTA -3′ | |||
| GR | DQ227738 | 102 | F : 5′- CTTCCATCCGCCCTTCA -3′ |
| R : 5′- TCGCATCTGTTTCACCC -3′ | |||
| MR | NM_001159345.1 | 210 | F: 5′- ACGCAGGATATGACAGCTCG-3′ |
| R: 5′- AGTACAGGGGCTTGGCATTC-3′ | |||
| 11β-HSD1 | XM_417988.2 | 229 | F: 5′-GGTGGTGAAAGAGGCTGAGAAC-3′ |
| R: 5′-GGAGGCGACTTTACCTGAAACAG-3′ | |||
| 11β-HSD2 | XM_003209680.1 | 229 | F: 5′-GGTGGTGAAAGAGGCTGAGAACA-3′ |
| R: 5′-GGAGGCGACTTTACCTGAAACAG-3′ | |||
| 20-HSD | NM_001030795.1 | 220 | F: 5′- CATCCTGAGAAGATAATGTCCAACG -3′ |
| R: 5′- TGCTTTGCAGATCATCAATATCCAG -3′ | |||
| 5-HT1A | GU189388.1 | 202 | F: 5′- AGAACACGGAGGCCAAGC -3′ |
| R: 5′- ACGGCAACCAGCAGAGGA -3′ | |||
| 5-HT1B | GU385013.1 | 133 | F: 5′- CACGGACCACGTCCTCTACAC -3′ |
| R: 5′- TTTCTTTGGCGTCTGCTTCA -3′ |
Table 1: Real-time PCR primers.
Statistical analysis
Descriptive statistics was performed to check the normality and homogeneity of variances before using parametric analyses. The behavioral data were not normally distributed, so Log 10 transformation was performed before statistical analysis. Body weight was analyzed by repeated measures ANOVA in the General Linear Model (GLM) procedure of SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Behavioral and plasma CORT, as well as the relative quantitative data of gene expression were analyzed by one-way ANOVA using SPSS 16.0 for Windows, followed by a least-significant difference (LSD) test for individual comparisons. A P-value ≤0.05 was considered significant.
Growth rate
In ovo injection of CORT significantly (P<0.05) affected the posthatch growth rate of the chickens. The chickens exposed to high doses of CORT grew slower compared to those in the low dose and control groups. As a result, high CORT birds did not weigh as much by the age of 7 weeks (Figure 1A).
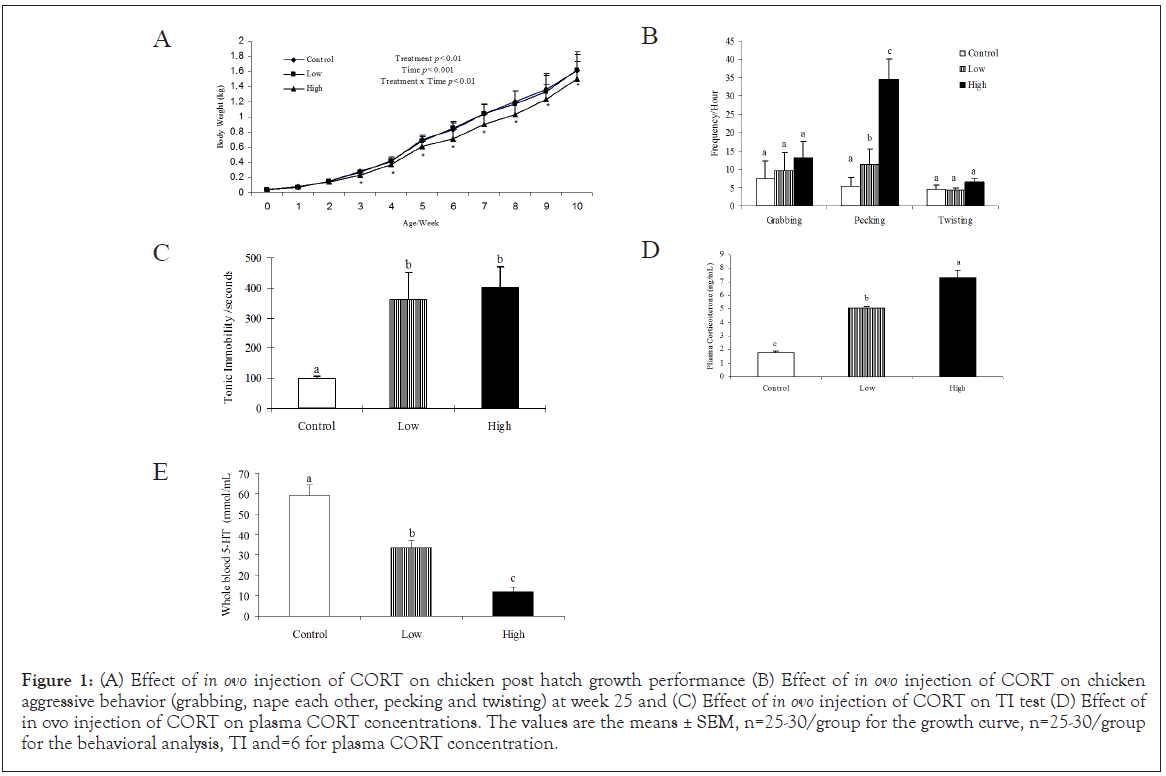
Figure 1: (A) Effect of in ovo injection of CORT on chicken post hatch growth performance (B) Effect of in ovo injection of CORT on chicken aggressive behavior (grabbing, nape each other, pecking and twisting) at week 25 and (C) Effect of in ovo injection of CORT on TI test (D) Effect of in ovo injection of CORT on plasma CORT concentrations. The values are the means ± SEM, n=25-30/group for the growth curve, n=25-30/group for the behavioral analysis, TI and=6 for plasma CORT concentration.
Aggressive behavior test and tonic immobility test
CORT administration significantly (P<0.05) increased the frequency of pecking behavior compared to control. The high dose of CORT significantly increased pecking behavior compared to the low group. No significant differences were observed in grabbing and twisting behaviors (Figure 1B). CORT administration significantly (P<0.05) increased TI duration both in low and high doses of CORT compared to the control group (Figure 1C). No significant differences were found between the male and the female chickens in the frequency of aggressive behaviors or tonic immobility (data not shown).
CORT concentrations in plasma
The high dose of CORT significantly (P<0.05) increased plasma CORT concentration compared to the low dose and control groups. However, the low dose of CORT treatment did not change plasma CORT concentrations (Figure 1D).
Hypothalamic 11β-HSD1, 11β-HSD2, 20-HSD, GR and MR mRNA expression
The high dose of CORT treatment significantly increased (P<0.05) hypothalamic 11β-HSD1 mRNA expression (Figure 2A) whereas, it decreased 11β-HSD2 mRNA (Figure 2B). In addition, both CORT treatments significantly (P<0.05) decreased MR mRNA expression in the hypothalamus compared to control (Figure 2E). CORT in ovo did not change neither 20-HSD mRNA (Figure 2C) nor GR (Figure 2D) expression in the hypothalamus.
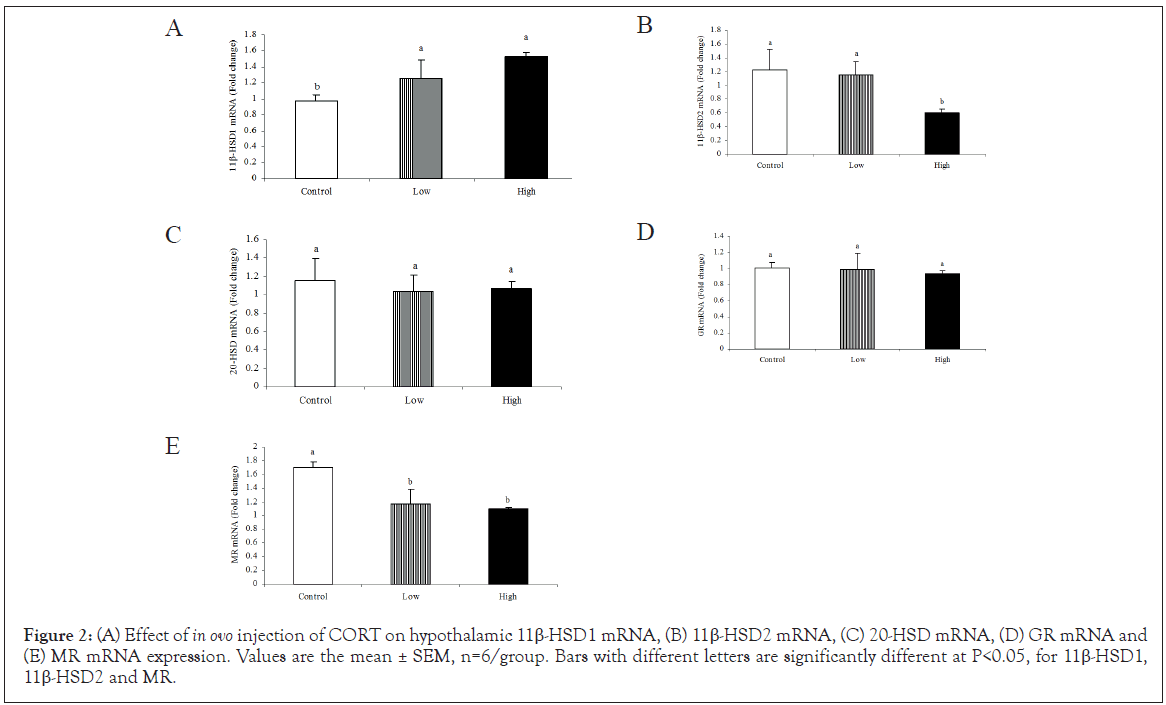
Figure 2: (A) Effect of in ovo injection of CORT on hypothalamic 11β-HSD1 mRNA, (B) 11β-HSD2 mRNA, (C) 20-HSD mRNA, (D) GR mRNA and (E) MR mRNA expression. Values are the mean ± SEM, n=6/group. Bars with different letters are significantly different at P<0.05, for 11β-HSD1, 11β-HSD2 and MR.
Hypothalamic 5-HTR1A and 5-HTR1B mRNA expression
The high dose CORT treatment significantly (P<0.05) increased the hypothalamic expression of 5-HTR1A mRNA compared to the low dose and control groups (Figure 3A). However, CORT treatment did not affect the 5-HTR1B mRNA expression in the hypothalamus (Figure 3B).
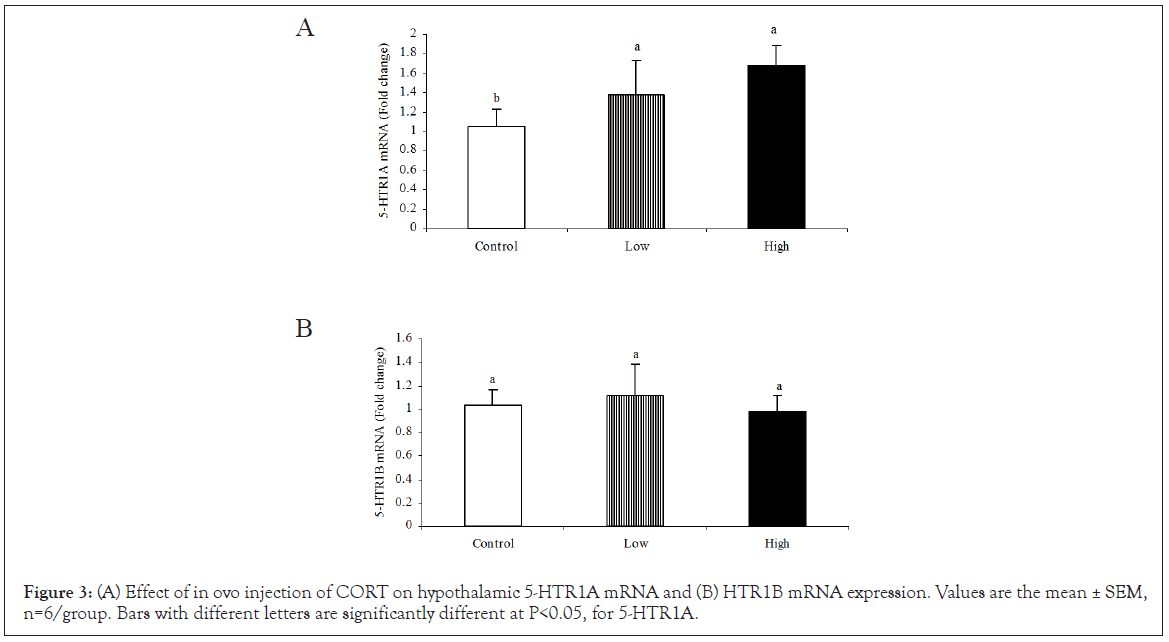
Figure 3: (A) Effect of in ovo injection of CORT on hypothalamic 5-HTR1A mRNA and (B) HTR1B mRNA expression. Values are the mean ± SEM, n=6/group. Bars with different letters are significantly different at P<0.05, for 5-HTR1A.
Ecological factors, such as maternal stress during the embryonic development increases the aggressive behavior of their offspring in rats and humans [77,78]. In avian species, egg CORT elevation was found to increased aggression in captive black-legged kittiwake [73] and increased pecking activities in domestic fowl [20]. In the present study, the in ovo injection of high dose of CORT significantly decreased growth rate in post-hatch chicks associated with plasma CORT concentrations. The increases of plasma CORT levels this study could be linked and be the cause of growth retardation. These findings in line with previous reports that embryonic CORT exposure retarded the growth in chickens [69] and quail [8], and increased plasma CORT concentration in the chickens [14,70]. Exposure to dexamethasone potent synthetic GCs (That induced comparable effects to CORT) retarded growth rate through the inhibition of protein biosynthesis in Japanese quail embryos [79] in addition inhibition of growth hormone releases from pituitary cultured cells [80]. In ovo CORT treatment elevated the circulating levels of CORT in hatch time in chickens [81] and at 8 weeks old in Japanese quail [82]. Similar results were reported in rats, revealing that exposure of CORT during gestation period inhibited fetal growth rate in mice [83,84] and permanently increased the plasma CORT levels in adult rats [85,86].
Prenatal stress has been found to promote aggressive behavior in humans [87,88]. In European starlings, the artificial elevation of yolk CORT enhanced offspring flight behaviors performance [18], increased fearfulness behavior in chickens [14], enhanced recall of a passive avoidance task in chickens [19], and increased the pecking behavior and pebbles in chickens [20]. In seabirds, the implanted CORT caused increases in the aggressive behavior when compared to the controls [89]. In agreement with these results, we found increased frequency of tonic immobility and aggressive behaviors in chickens that prenatally exposure to the high dose of CORT.
Prenatal stress during late gestational period causes alterations in 11β-HSD1 mRNA expression in marmosets [90], human [91], rodents [92], and decreased 11β-HSD2 mRNA expression in later life in mammals (Review) [93]. In agreement with those findings, High dose administration of CORT upregulated hypothalamic 11β-HSD1 expression whereas, down regulated 11β-HSD2 mRNA in the hypothalamus. In mammalian species, up regulation of hippocampal 11β-HSD1 mRNA expression together with cerebral cortex resulted in decline of cognitive which was associated with aging in mice [94]. In humans and rodents, 11β-HSD1 over expression was associated with obesity in later life [95]. The reduction in the activity or expression of 11β-HSD2 during pregnancy was resulted in development of metabolic syndromes [96] such as hypertension [97,98], glucose intolerance [99] as well as the programming of HPA axis activity [100] and anxiety related behaviors in later life [42,101,102].
In this study, the increases of aggressive behaviors in exposed high CORT chickens were associated with alterations in the hypothalamic expression of serotonergic genes mRNA. A significant increase was found in hypothalamic 5HTR1A mRNA expression in response to CORT treatment. There are several lines of evidence indicating that the neurocircuits for stress and aggression are reciprocally interrelated in non-mammalian species [103-105]. Animals predisposed to be dominant often show higher GCs associated with lower 5-HT [46]. GCs and prenatal stress were reported to enhance 5-HT system function and increase 5-HTR1A mRNA expression in rat [106,107], which seems to agree with our current finding. However, it is remain unknown whether these effects will be transmitted to the next generation in chickens, thus, the stressed mother that deposited high amounts CORT in the eggs may have similar phenotypic and behavioral outcomes.
In conclusion, our findings suggest that prenatal CORT exposure may influence the phenotype, aggressive behavior and tonic immobility of chickens. Changes in hypothalamic GCs metabolic enzymes and 5-HT system gene expression could be due to the consequence of earlier effects on growth retardation. Further studies are required to clarify the transgenerational effect of CORT in ovo in chickens.
No conflict of interests exists.
This work was supported by the NSFC-Guangdong Joint Fund (Project No. U0931004), the Special Fund for Agro-scientific Research in the Public Interest (201003011), and the Priority Academic Program Development of Jiangsu Higher Education Institutions. Especial thanks conducted to Professor Donald C. Lay his critical revision of manuscript.
Citation: Ahmed AA, Essa MEA, Mollica A, Stefanucci A, Zengin G, Ahmed H, et al. (2020) Prenatal Corticosterone Exposure Alters Glucocorticoid Metabolic Enzyme Eene mRNA Associated with Increased Aggressive Behaviors and Tonic Immobility in Chicken. J Clin Cell Immunol. 11:604.
Received: 29-Sep-2020 Accepted: 13-Oct-2020 Published: 20-Oct-2020 , DOI: 10.35248/2155-9899.20.11.604
Copyright: © 2020 Ahmed AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.