
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2025)Volume 8, Issue 1
Investigation of the value of Artificial Intelligence (AI) quantitative parameters combined with Circulating Tumor Cell testing (CTC) in lung adenocarcinoma infiltration.
Methods: Images of 127 surgically confirmed samples of lung adenocarcinomas were analyzed retrospectively between January 2020 and December 2022, and based on postoperative pathology, the lung adenocarcinomas were divided into the non-infiltrating group (65 cases) and the infiltrating group (62 cases), with the latter, including ICA. Five sets of quantitative indices, namely, the longest diameter, volume, mass, mean CT value, and maximum CT value, of each nodule, was analyzed using AI analysis software. The patients were subjected to the CTC detection test prior to the surgery. The differences in the above five quantitative indices between the two groups were determined, following which the ROC curve analysis and calculations for the Area Under the Curve (AUC), 95% CI, sensitivity, specificity, critical value, and compliance rate were performed for each group.
Results: The quantitative indices (longest diameter, volume, mass, maximum CT and mean CT of each nodule) were higher in the infiltration group compared to the non-infiltration group, and the difference between the two groups was statistically significant (P<0.05). The number of CTC-positive cases was higher in the infiltration group compared to the non-infiltration group, and the difference was statistically significant (P<0.05). The Area Under the Curve (AUC) for the longest diameter, volume, mass, maximum CT, and mean CT was 0.845, 0.850, 0.756, 0.727, and 0.871, respectively. The highest sensitivity for each quantitative parameter was obtained as 88.7% for CTC, the highest specificity was 93.8% for maximum CT, and the highest compliance was 83.5% for maximum CT. The volume, mean CT, and CTC were revealed as independent risk factors for predicting the infiltrative nature of pulmonary nodules, with the respective Odds Ratio (OR) of 1.001, 1.006, and 5.065; the corresponding 95% CI were 1.000-1.001, 1.002-1.009, and 1.269-20.210, respectively, with P<0.05. The mean value of the AUC of the combined model was 0.934, with 95% CI in the range of 0.887 to 0.982, a sensitivity of 91.9%, a specificity of 87.7%, and a compliance rate of 88.20%.
Conclusion: The quantitative AI parameters of volume and mean CT value combined with CTC provide a better preoperative prediction of the infiltrative nature of lung adenocarcinoma.
Artificial intelligence; Quantitative parameters; Circulating tumor cell detection; Lung adenocarcinoma
The number of lung nodules detected in the residents of China has increased dramatically in recent years due to the increasing awareness of health among Chinese citizens and the promotion of low-dose chest CT for lung cancer screening in the nation [1]. According to the TNM staging of lung cancer, early-stage lung adenocarcinoma is classified into Atypical Adenomatous Hyperplasia (AAH), Adenocarcinoma In situ (AIS), Minimally Invasive Adenocarcinoma (MIA), and Invasive Adenocarcinoma (IAC), based on the degree of infiltration of the tumor cells [2,3]. However, predicting the infiltrative nature of lung cancer prior to surgery remains a challenge for clinicians and imaging physicians. Currently, the CT analysis of lung nodules focuses primarily on revealing the morphological signs (margins, tumorpulmonary interface, endocarcinoma nodules, etc.) and nodule size, density, and imaging histology [2,4]. A limited number of studies have explored the combined use of quantitative Artificial Intelligence (AI) parameters and Circulating Tumor Cells (CTC) as indicators of the presence of lung nodules. In this context, the present study investigated the application of quantitative AI parameters combined with CTC to predict the infiltration of lung adenocarcinoma, and the findings are expected to assist in the clinical diagnosis and treatment of lung adenocarcinoma.
Case data
Clinical, imaging, and pathological data were collected from 127 patients who underwent the surgical resection of pulmonary nodules between January 2020 and December 2022 at Zhongshan Hospital of Traditional Chinese Medicine. The inclusion criteria were as follows: 1) Chest CT examination and CTC examination were performed one week prior to the surgical resection of lung nodules; 2) Lung adenocarcinoma was confirmed surgically; 3) The longest diameter of the nodule was ≤ 30 mm; 4) The data were imported into an AI system and all images were recognized. The exclusion criteria were as follows: 1) Incomplete clinical data or CT images or CTC examination; 2) Chemotherapy or radiotherapy was performed prior to the CT examination and surgical resection. A total of 127 patients, aged between 25 and 80 years, with a median age of 58 years, were finally included in the present study. According to the results of surgical pathology, AAH, AIS, and MIA were included in the non-infiltration group (65 cases) and ICA was included in the infiltration group (62 cases). The included cases comprised 51 cases of pure groundglass nodules, 52 cases of mixed ground-glass nodules, and 24 cases of solid nodules, which were from a total of 55 males (29 cases in the non-infiltration group and 26 cases in the infiltration group) and 72 females (36 cases in the noninfiltration group and 36 cases in the infiltration group). The CTC examination was positive in 79 cases (25 cases in the noninfiltration group and 54 cases in the infiltration group) and negative in 48 cases (40 cases in the non-infiltration group and 8 cases in the infiltration group). The study was designed as a retrospective study and was approved by the ethics committee of the hospital. Informed consent from the patients was not required.
Examination methods
Chest CT examination: CT examination was performed using a Philips iCT 256-row spiral CT scanner or a Toshiba Acquillion 64-row spiral CT scanner, with a scanning range of the entire area from the lung tip to the lung base. The scanning parameters used were as follows: Tube voltage, 120 kV; tube current, 110– 180 mA; layer thickness, 5 mm; Field of View (FOV), 35 cm × 35 cm; matrix, 512 × 512; lung algorithm, standard algorithm reconstruction. The image reconstruction parameters were a layer thickness of 1 mm and a reconstruction layer spacing of 1 mm.
AI software analysis: The DICOM format images (layer thickness of 1 mm and layer spacing of 1 mm) of 127 chest reconstructed lung windows were imported one by one into the CT image-assisted detection software for lung nodules (ROO1) from United Imaging for conducting the quantitative analyses. The center of the lung nodule was selected manually and marked. The AI software could initially identify the boundaries of the lung nodules; most of which were identified satisfactorily, and the cases of unsatisfactory identification of the boundaries were edited manually and outlined on a level-by-level basis. The outlining process attempted to exclude areas such as blood vessels, bronchi, and lung atelectasis. Afterward, the AI software automatically analyzed five groups of quantitative indices, namely, the longest diameter, volume, mass, mean CT, and maximum CT, of each nodule (Figures 1-4). All images were outlined by two physicians with experience of 5 years and 12 years, respectively, in diagnostic chest imaging. The outlining condition used by the physicians was the lung window of –700 HU, 1600 HU.

Figure 1: Non-infiltration group (male, 61 years old, AIS); a) Thin transverse bitmap of a pure ground glass nodule in the upper lobe of the right lung; b) Quantitative data: nodule volume 176.97 mm3, mass 54.79 mg, CT max –560, CT homogeneity -690.4; c) Pathological image captured microscopically, revealing the adenocarcinoma in situ (×100, HE).
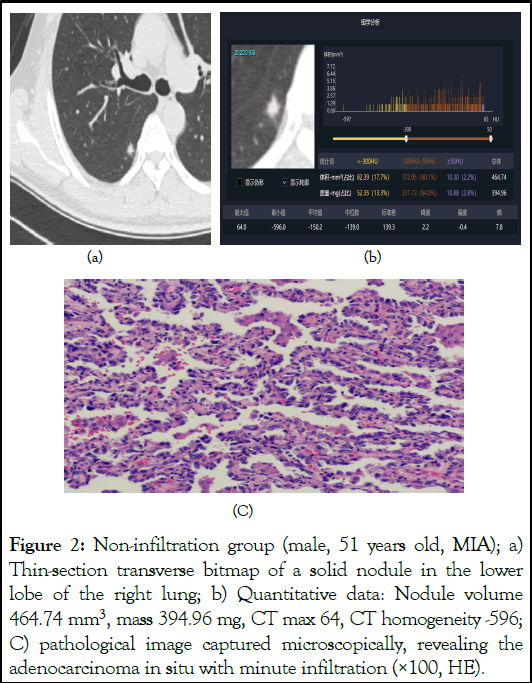
Figure 2: Non-infiltration group (male, 51 years old, MIA); a) Thin-section transverse bitmap of a solid nodule in the lower lobe of the right lung; b) Quantitative data: Nodule volume 464.74 mm3, mass 394.96 mg, CT max 64, CT homogeneity -596; C) pathological image captured microscopically, revealing the adenocarcinoma in situ with minute infiltration (×100, HE).

Figure 3: The infiltration group (female, 75 years old); a) Thin section transverse bitmap of a solid nodule in the lower lobe of the right lung, b) Quantitative data: nodule volume 3089.96 mm3, mass 2812.30 mg, CT max value 177.0, CT uniform value –899; C) Pathological image captured microscopically, revealing the pulmonary invasive adenocarcinoma (predominantly the papillary type, approximately 70%; micropapillary type, approximately 10%; predominantly the vesicular type, approximately 10%; adnexal type, approximately 10%) (×100, HE).
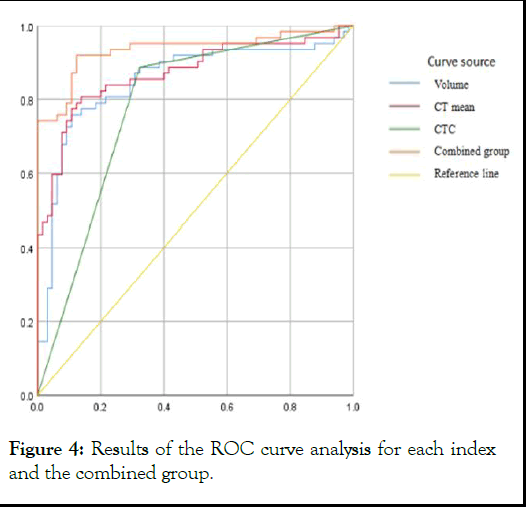
Figure 4: Results of the ROC curve analysis for each index and the combined group.
CTC detection: The CTC negative enrichment purification system was employed for the CTC enrichment of lung cancer preoperative blood samples. The cells were identified and labeled with a specific Folate Receptor α (FRð) nucleic acid probe. The results were quantified through PCR using a FRð probe, specific primers, and an oligonucleotide fluorescent probe, and the results were expressed in the units of FU/3 mL. The CTC assay reagents were purchased from Jiangsu Lail Bio-medical Science and Technology Co. Ltd.
Statistical analysis
The statistical software IBM SPSS 26.0 and MedCalc20.027 were employed for statistically analyzing the result data. The independent sample t-test was performed to compare two groups of data for normally distributed measures. Mann-Whitney U-test was performed to compare two groups of data for non-normally distributed measures. The chi-squared test or Fisher's exact probability method was adopted to compare the different groups of categorical variables. ROC curves were utilized to analyze the diagnostic efficacy of quantitative CT parameters. A multivariate logistic regression analysis was performed to establish a prediction model for distinguishing the non-infiltrating group from the infiltrating group, and the Odds Ratio (OR) was calculated. The Hosmer-Leme-show test was then performed to evaluate the goodness of fit of the established model. The diagnostic efficacy of the combined model was compared based on the volume, mean CT, and CTC using ROC curves and Ztests, and differences were considered statistically significant at P<0.05.
Clinical data
No statistically significant differences were observed in terms of gender, age, and smoking between the non-infiltration group and the infiltration group (P>0.05). The basic clinical data of the patients in these two groups are provided in Table 1.
| Indicators | Non-infiltrating group (n=65) | Infiltration group (n=62) | t/X2/Z value | P value |
|---|---|---|---|---|
| Gender | 0.930a | 0.761 | ||
| Male | 29 (44.6%) | 26 (41.9%) | ||
| Female | 36 (55.4%) | 36 (58.1%) | ||
| Age (years old) | 58.00 (51.02, 57.57) | 58.50 (55.78, 61.60) | 52.403b | 0.24 |
| Smoking (number of cases) | 11 (16.9%) | 10 (16.1%) | 0.398b | 0.82 |
| Note: a is the X2 chi-square value from the chi-square test or Fisher's exact probability method test; b is the Z value from the Mann-Whitney U test. | ||||
Comparison of CTC detection and CT quantitative indices between the two groups
The longest diameter, volume, mass, maximum CT, and mean CT of the nodules did not follow a normal distribution, and, therefore, the Mann-Whitney U test was performed to compare the data between the two groups. The quantitative indices (the longest diameter of the nodule, volume, mass, maximum CT, and mean CT) were higher in the infiltration group compared to the non-infiltration group, and the difference between the two groups was statistically significant (P<0.05). The number of CTC-positive cases in the infiltration group was higher than that in the non-infiltration group, and the difference was statistically significant (P<0.05). A comparison of the CTC detection results and CT quantitative indices between the two groups is presented in Table 2.
| Indicators | Non-infiltrating group (n=65) | Infiltration group (n=62) | t/X2/Z value | P value |
|---|---|---|---|---|
| Longest diameter (mm) | 11.00 (10.64, 12.71) | 19.50 (17.35, 20.33) | -6.716a | 0 |
| Volume (mm3) | 581.24 (619.89, 1298.00) | 3064.86 (3183.16, 5468.28) | -6.801a | 0 |
| Mass (mg) | 663.83 (713.55, 1091.19) | 1470.37 (1461.64, 2152.65) | -4.982a | 0 |
| Maximum value of CT (HU) | -36 (-72.20, -35.15) | 32.00 (-14.01, 19.78) | -4.416a | 0 |
| Mean value of CT (HU) | -565.30 (-582.94, -509.08) | -160.35 (-282.94, -172.67) | -7.211a | 0 |
| CTC | 42.006b | 0 | ||
| Negative | 44 (67.7%) | 7 (11.3%) | ||
| Positive | 21 (32.3) | 55 (88.7%) | ||
| Note: a is the z-value from the Mann-Whitney U test; b is the X2 chi-square value from the chi-square test or Fisher's exact probability method test | ||||
Table 2: Comparison of the quantitative indicators and CTC between the non-infiltration and infiltration groups.
ROC curve analysis of each quantitative parameter
Separate ROC curve analyses were performed for each quantitative parameter and CTC, which revealed the Area Under the Curve (AUC) values of 0.845, 0.850, 0.756, 0.727, and 0.871 for the longest diameter, volume, mass, maximum CT, and mean CT, respectively. The highest sensitivity for each quantitative parameter was 88.7% for CTC, the highest specificity was 93.8% for the maximum CT value, and the highest compliance rate was 83.5% for the maximum CT value. The ROC curves of each quantitative parameter are presented in Table 3. The subsequent multifactorial binary logistic regression analysis of each quantitative parameter and CTC revealed volume, mean CT value, and CTC as independent risk factors for the prediction of nodal infiltration, with the respective ORs of 1.001, 1.006, and 5.065, and 95% CI of 1.000–1.001, 1.002– 1.009, and 1.269–20.210, respectively, with P<0.05.
| Parameter | AUC | 95% CI | Sensitivity | Specificity | Threshold | Compliance rate |
|---|---|---|---|---|---|---|
| Longest diameter (mm) | 0.845 | 0.770~0.920 | 79.00% | 86.20% | 14.5 | 78.70% |
| Volume (mm3) | 0.85 | 0.777~0.922 | 75.80% | 89.20% | 1511.3 | 79.50% |
| Mass (mg) | 0.756 | 0.669~0.844 | 87.10% | 56.90% | 741.72 | 69.30% |
| Maximum value of CT | 0.727 | 0.637~0.817 | 51.60% | 93.80% | 22.5 | 83.50% |
| Mean value of CT | 0.871 | 0.806~0.936 | 80.60% | 86.20% | -428.4 | 82.70% |
| CTC | 0.782 | 0.699~0.865 | 88.70% | 67.70% | -a | 78.00% |
| Joint model | 0.934 | 0.887~0.982 | 91.90% | 87.70% | -b | 88.20% |
| Note: The combined model contained 3 variables, namely, CTC, mean CT, and volume; a) the corresponding indicator is a dichotomous variable with no critical value; b) the corresponding indicator is the predicted probability with no critical value. | ||||||
Table 3: Results of the analysis of individual quantitative parameters, CTC, and ROC curves of the combined group.
Diagnostic efficacy analysis of the combined model
The volume, mean CT, and CTC were used in combination to establish a combined model for predicting the diagnosis of infiltrative lung nodules using multifactorial logistic regression analysis. The established combined diagnostic model predicted the following hazard score formula for infiltrative lung nodules: Logit(P)=–1.192+0.001 × volume+0.006 × mean CT+1.821 × CTC.
The Hosmer-Lemeshow test revealed that the combined model had a good fit (P=0.195). The mean AUC of the combined model was 0.934, with a 95% CI of 0.887–0.982, sensitivity of 91.9%, specificity of 87.7%, and a compliance rate of 88.20%. The diagnostic efficacy of the combined model was significantly higher than that of volume, mean CT, and CTC used independently for prediction (Z=2.315, 2.290, and 4.7, respectively; all P-values were <0.05).
Only the cases of AAH and AIS with close follow-up or planned local excision could achieve 100% survival at 5 years. The 5-year survival rate of MIA with timely detection and surgery was close to 100% [5]. Patients with IAC were susceptible to lymph node metastasis and hematogenous metastasis, with the highest 5-year survival rate of just 60% to 80% [6]. According to the 5-year survival rates, the cases of AAH, AIS, and MIA were grouped into the non-infiltration group while IAC cases were grouped into the infiltration group in the present study.
The predictive value of the quantitative AI parameters in lung adenocarcinoma invasiveness
The detection of lung nodules using AI algorithms, which is a current hotspot in the research and development of AI medical devices [7,8], would assist physicians in rapidly and accurately detecting lung nodules, thereby determining the risk of lung cancer at an early stage and enhancing the efficiency and role of physical examination and screening in the prevention and treatment of lung cancer, ultimately facilitating the early detection, early diagnosis, and early treatment of lung cancer [9]. In lung nodule diagnosis, AI could effectively distinguish between lung nodules and non-nodules, exhibiting a reduced false-positive rate and an increased rate of detection of lung nodules [10]. In addition, when using AI, the three-dimensional texture features, the clinical information, and the CT imaging data of the lung nodules are counted into the support vector machine model for lung cancer prediction which could improve the sensitivity and specificity of diagnosis by radiologists.
In the present study, the AI analysis software for lung nodules was employed to quantify five indices, namely, the longest diameter, volume, mass, maximum CT, and mean CT of each lung nodule, for the pathological diagnosis of AAH, AIS, MIA, and IAC. One-way regression analysis revealed that these five quantitative indices of lung adenocarcinoma were higher in the infiltration group compared to the non-infiltration group, and the difference between the two groups was significant (P<0.05), which was consistent with the findings of Cao [11]. A further multifactorial logistic regression analysis revealed volume, mean CT, and CTC as independent risk factors for predicting the infiltrative nature of pulmonary nodules.
Most of the existing studies have predicted the progression of pulmonary nodules using the volume of the nodule versus the mean CT value [12]. The present study revealed that the volume and mean value of CT were independent risk factors for predicting the infiltrative nature of pulmonary nodules. In addition, it was demonstrated that volume had a high diagnostic efficacy, as evidenced by its AUC of 0.850 and a critical value of 1511.30 mm3. Volume also presented a higher specificity and compliance rate compared to the longest diameter of the nodule. In the author’s opinion, several factors could have led to the higher diagnostic efficacy of the volume of pulmonary nodules compared to that of the longest diameter and area. It is known that the development of lung adenocarcinoma generally follows the AAH-AIS-MIA-ICA process, in which the tumor cells grow along the alveolar wall, and as the tumor progresses, the number of tumor cells and infiltration increase, ultimately resulting in a significantly enlarged tumor, and at certain times, the tumor does not present a significant increase in the longest diameter while a significant increase in its volume is observed.
According to studies, the size and density of lung nodules may be used for predicting the degree of malignancy of lung cancer [13,14]. The different density ranges and thresholds are also suggested to be useful in differentiating between different pathological types [15], with IAC having a larger diameter and density than AIS and MIA [16]. Kitami reported that pure ground glass nodules with a mean CT value of –600 HU could be used as a clinical indicator of pre-invasive and invasive lesions. However, certain authors reported that the mean CT of –520 HU distinguishes MIA from AAH and AIS [17,18]. The present study demonstrated that the mean CT value of –428.40 HU served as the best threshold for distinguishing infiltration from non-infiltration. The thresholds revealed in the present study were higher than those reported in the above-stated two previous studies, which were attributed to the different subgroups used in the studies. While the present study included AAH, AIS, and MIA in the non-invasive group and IAC in the invasive group, Kitami's study included AAH and AIS in the non-invasive group and MIA and IAC in the invasive group. In addition, Kitami's study included only ground glass nodules (pure and mixed ground glass nodules), while the present study included solid nodules in addition to ground glass nodules.
The predictive value of CTC in lung adenocarcinoma invasiveness
CTCs are tumor cells that enter the peripheral blood circulation. Certain studies have confirmed that CTC has a higher sensitivity than the traditionally used markers of lung cancer and tumors in the adjuvant diagnosis of lung nodules [19]. CTCs have been detected in the peripheral circulation of patients with early-stage lung cancer. China was the first nation to report the use of the FRα-targeted Polymerase Chain Reaction (PCR) method for CTC detection. FRα is tumor-specific and expressed highly on the surface of lung cancer tumor cells while a much lower expression is observed in non-malignant tumor tissues [20]. Preoperative CTC of pulmonary nodules reportedly enables differentiating between AIS and MIA. CTC level, therefore, has potential predictive value in determining the presence of aggressive histologic patterns (micropapillary, solid, and advanced subtypes), degree of differentiation, and occurrence of visceral pleural invasion and lymph node metastasis in IAC. The present study demonstrated that CTC positivity was statistically significant in the 55 cases of the infiltration group (88.7%) and also in the 21 cases of the non-infiltration group (32.3). CTC emerged as a good predictor of lung adenocarcinoma infiltration, with ROC: AUC=0.782, sensitivity=88.7%, specificity=67.7%, and compliance rate=78.0%.
The predictive value of AI quantitative parameters combined with CTC in lung adenocarcinoma invasiveness
CT is currently used as the main diagnostic method for early lung cancer, although its accuracy in diagnosing the benign and malignant nature of isolated lung nodules with a diameter of <10 mm, particularly those with a diameter of <5 mm, is low. It has been long established that blood vessels may form within the tumor when the diameter of the tumor exceeds 2 mm, to provide nourishment to the tumor, which may subsequently facilitate tumor cell detachment and entry into circulation. A study demonstrated that a tumor size of less than 1 mm could predict the presence of CK and HER2-expressing tumor cells in the circulating blood and, therefore, the isolated pulmonary nodules with a diameter <5 mm were also likely to produce CTCs. The results of Mitra indicated 100% specificity and 89% sensitivity of circulating tumor cell screening using a circulating tumor cell count of greater than 25 as the threshold, thereby filling the gap in the application of imaging in the diagnosis of lung nodules smaller than 5 mm.
In the present study, the three independent risk factors, namely, the nodule volume, mean CT, and CTC, were subjected to multifactorial logistic regression analysis to establish a combined diagnostic model for the prediction of pulmonary nodule infiltration. The ROC diagnostic efficacy of the combined model was higher than the independent application of each of the three factors. Ma reported CTC and AI tumor volume as independent indicators of the invasiveness of the pathological subtypes of lung adenocarcinoma.
In summary, the quantitative AI parameters, namely, the nodal volume and the mean CT value, combined with CTC, are expected to provide a better preoperative prediction of the invasiveness of lung adenocarcinoma.
In conclusion, this study explored the integration of quantitative Artificial Intelligence (AI) parameters and Circulating Tumor Cell (CTC) testing for predicting the infiltrative nature of lung adenocarcinoma. The findings underscore several key points:
In summary, the study supports the use of quantitative AI parameters combined with CTC testing as a promising approach for predicting the invasiveness of lung adenocarcinoma. This integrated method holds potential to advance personalized medicine in lung cancer management by refining risk assessment and guiding tailored treatment strategies.
This study was supported by the grant from Zhongshan City Social Welfare Science and Technology Research Project (2022B142) and Scientific Research Fund of Hunan Provincial Education Department (Grant No.HNJG-2021–1216) and Provincial Natural Science Foundation of Hunan (Grant No. 2019JJ40202).
All authors contributed to the study conception and design. WM and YZ wrote the original draft and analyzed the data, acquired the funding and gave final approval of the version to be published. SL and MZ revised the article and prepared the tables and figures. ZC analyzed and interpreted the data.
This paper is funded by Zhongshan City Social Welfare Science and Technology Research Project (2022B142) and Scientific Research Fund of Hunan Provincial Education Department (Grant No.HNJG-2021–1216) and Provincial Natural Science Foundation of Hunan (Grant No.2019JJ40202).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare no competing interests.
The authors declare that they have no conflict of interest.
This study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board of our hospital
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Mao W, Li S, Zhou M, Zhang Y, Chen Z (2025) Predictive Value of Quantitative Artificial Intelligence Parameters Combined with Circulating Tumor Cell Detection in Lung Adenocarcinoma Infiltration. J Clin Chem Lab Med. 8:301.
Received: 25-Oct-2023, Manuscript No. JCCLM-23-27773; Editor assigned: 27-Oct-2023, Pre QC No. JCCLM-23-27773 (PQ); Reviewed: 10-Nov-2023, QC No. JCCLM-23-27773; Revised: 08-Jan-2025, Manuscript No. JCCLM-23-27773 (R); Published: 15-Jan-2025 , DOI: 10.35248/2736-6588.25.8.301
Copyright: © 2025 Mao W, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.