Virology & Mycology
Open Access
ISSN: 2161-0517
ISSN: 2161-0517
Research Article - (2021)Volume 10, Issue 3
Potential of Trichoderma harzianum for biological control of postharvest fungal rot of white yam (Dioscorea rotundata Poir) tubers in storage was studied. Pathogenicity test revealed the susceptibility of healthy looking yam tubers to Aspergillus niger, Botryodiplodia theobromae and Fusarium oxysporum f. sp. melonganae after fourteen days of inoculation. Treatments comprising A. niger, B. theobromae and F. oxysporum each paired with T. harzianum and were arranged in completely randomized design and stored for five months between December, 2015 and April, 2016 and December, 2016 and April, 2017. Results revealed that tubers treated with the pathogenic fungi alone caused mean percentage rot of between 6.67% (F. oxysporum) and 22.22% (A. niger) while the paired treatments produced only between 2.22% (T. harzianum X F. oxysporum) and 6.67% (T. harzianum X A. niger). In the second year of storage, mean percentage rots were between 13.33% (F. oxysporum) and 28.89% (A. niger) while in the paired treatment rots were only between 6.67% (F. oxysporum X T. harzianum) and 8.89% (A. niger X T. harzianum). Tubers treated with antagonist alone produced 0.00% and 2.22% in the first and second year respectively. Result revealed that there was a significant difference (P ≤ 0.05) in mean percentage rot between the first year and the second year except where B. theobromae was inoculated alone, A. niger and T. harzianum paired and B. theobromae and T. harzianum paired. The most antagonized fungus in paired treatment for both years was F. oxysporum f. sp. melonganae while the least antagonized was A. niger and B. theobromae. The study recommended the use of T. harzianum in the control of rot causing pathogens of yam tubers in storage as better alternative ways of reducing tuber rot compared with the use of chemical fungicides which are environmentally hazardous.
Biological control; Fungal rot; Postharvest; White yam; Trichoderma harzianum
Yams (Dioscorea spp.) are the most important tubers and food crops consumed by millions of people in different parts of tropical and sub- tropical countries in Africa, Caribbean, The Northern and Central part of South East Asia including parts of China, Malaysia, Japan and Oceania [1-3]. Consumption of yam may be by preparation of varieties of palatable dishes from yam tubers [4,5]. The principal food nutrient in yam is mostly carbohydrate and low content of protein as well as ascorbic acid [6]. Yam tubers are affected by different pathogens both in the field and in storage which reduce the quantity and market value of the tubers [7]. Studies conducted in different parts of Nigeria have shown that the greatest causes of yam tuber loss in storage are fungal rot organisms [8,9]. These pathogenic organisms included: B. theobromae Pat., F. oxysporum Schlencht, Penicillium oxalicum Currie and Thom, Sclerotium rolfsii Sacc, A. niger Van Tiegh and A. tamarii Kita [7,10-12]. Several methods have been adopted for controlling losses due to post harvest disease of yam; these include the use of chemicals such as captan, benomyl, thiobendazole and mancozeb which have been found to significantly inhibit the growth of rot causing organisms [10,13,14]. Effects of chemicals on the environment have been found to be detrimental [15]. Biological method of control using T. viride, T. harzianum, Pseudomonas syringae, P. chlororaphis, have been found to be effective in controlling postharvest pathogens of different crops [16-18]. T. harzianum is a filamentous soil fungus known to be an effective biocontrol agent for a range of important airborne and soil borne pathogens. Trichoderma spp. is the most widely studied Bio Control Agents (BCAs) against plant pathogens [19]. The parasitic activity of T. harzianum is mediated by its excretion of a variety of enzymes including cellulases, chitinases and antibiotics such as gliotoxin. Trichoderma spp are economically important because of their mycoparasitic ability and strong aggressiveness which make them suitable for application as biocontrol agents against soil-borne plant-pathogenic fungi [20-22]. The research therefore focuses on the antagonistic potential and biological control of T. harzianum in controlling yam fungal pathogens in storage.
Source of biological control agent
The biological control agent, T. harzianum was collected from yam pathological unit of University of Ibadan, Oyo State, Nigeria. Stock cultures of the isolate were aseptically prepared and maintained on slants of acidified Potato Dextrose Agar (PDA) in McCartney bottles and stored for subsequent studies.
Isolation and identification of fungal pathogens
Rotted yam tubers were collected from farmers’ storage barns and washed under running tap water to remove surface soil, debris and other contaminants. Small tissue pieces of approximately 2 × 2 mm were cut out from the leading edge of lesion with sterile scalpel and surface sterilized in 5% Sodium hypochlorite for 2 minutes, the pieces were then washed in four successive changes of sterile distilled water and dried on sterile filter paper [14]. The dried infected pieces were later aseptically plated on Petri dishes containing acidified sterile Potato Dextrose Agar (PDA). The inoculated plates were incubated at ambient room temperature (30 ± 5°C) for 7 days. Sub-culture of growing fungi mycelia were identified after 7 days of incubation when pure cultures were obtained [14]. Microscopic examination and morphological characteristics of the growing fungi colony were noted and compared with already established authorities [23,24].
Pathogenicity test
Healthy white yam tubers of Ogoja cultivar (Dioscorea rotundata) were washed under running tap water to remove soil. The tubers were surface sterilized by dipping each yam tuber into 5% concentration of sodium hypochlorite for 2 minutes and rinsed in four successive changes of sterile distilled water [14]. The tubers were then placed on sterile filter paper in the Laminar Air Flow Cabinet to dry for 30 minutes. A 5mm diameter cork borer was used to remove 4mm tissue from the healthy Ogoja white yam tuber surfaces aseptically [25]. A 5-mm diameter mycelial agar plug of a 5-day-old culture of A. niger, B. theobromae and F. oxysporum were used for inoculation. These fungal plugs were separately put in the holes created in the yam tubers. Petroleum jelly was used to seal the edges of the replaced yam tissues [26]. The same procedure was replicated for the control experiments except that discs of uninoculated potato dextrose agar were placed in the holes created in the tubers instead of the fungi mycelia [27]. The inoculated yam tubers were completely randomized [28] and incubated at ambient room temperature (30 ± 5°C) for 14 days under sterile condition to determine rot. When rot symptoms appeared, a sterilized and flamed knife was used to cut open the inoculated yam tubers from the point of inoculation to see the level of infectivity.
Preparation of fungal spore suspension and culture of T. harzianum
Fungal spores’ suspensions of A. niger, B. theobromae and F. oxysporum, and the antagonist, T. harzianum were prepared from 5 days old cultures grown on Potato Dextrose Agar (PDA) plates. Conidia from the surface of agar plate were scrapped with sterile glass rod to dislodge the spores [29] and re-suspended in 1L of sterile distilled water containing 5% Tween 80 [30]. The spore suspensions obtained were filtered through four folds layer of sterile cheesecloth into a sterile 1000ml Pyrex glass beaker. The suspension concentrations were determined by using an improved Neubauer haemocytometer (model BS 748) and adjusted to 1×106 spores per ml.
Determination of the interaction between rot fungi (A. niger,B.theobromaeand F.oxysporum) and biological antagonist (T. harzianum) when inoculated on healthy Ogoja white yam tubers
Healthy white yam tubers of Ogoja cultivar (D. rotundata) were first washed under running tap water to remove dirt and soil particles, before immersing in 5% Sodium hypochlorite solution for 2 minutes and rinsing again in four successive changes of sterile distilled water to remove surface contaminants [14]. The yam tubers were placed on filter papers to dry. Treatments comprising A. niger, B. theobromae and F. oxysporum each paired with T. harzianum separately were set up to determine their effects on rot in Ogoja white yam tubers. Yam tubers were also inoculated with each of the fungal isolates separately without T. harzianum. Yam tubers without fungal isolates and T. harzianum served as the control. T. harzianum was paired with the three pathogenic fungi and the Ogoja white yam tubers were inoculated separately according to the following inoculation regime as described [16]:
A. Uninoculated yam tubers (control);
B. Tubers inoculated with A. niger alone;
C. Tubers inoculated with B. theobromae alone;
D. Tubers inoculated with F. oxysporum alone;
E. Tubers inoculated with T. harzianum alone;
F. Tubers inoculated with T. harzianum and A. niger simultaneously;
G. Tubers inoculated with T. harzianum and B. theobromae simultaneously;
H. Tubers inoculated with T. harzianum and F. oxysporum simultaneously;
Three tubers formed a treatment; each of the eight treatments was replicated three times giving a total of nine tubers per treatment. 72 tubers of yams were examined in this experiment for the eight different treatments.
The suspension for each of the treatments was poured in a hand sprayer and the yam tubers were sprayed accordingly [31-33]. The yam tubers were arranged in completely randomized design and stored at ambient room temperature (30 ± 5 °C) for five months. The control tubers were sprayed with sterile distilled water before storage. Record of rotted tubers were kept on periodic basis and cumulative percentage rot during storage of yam tubers that were inoculated with T. harzianum and the post-harvest pathogens of yams in different combinations were evaluated based on the symptoms of rot on the tubers at monthly interval for five months between December, 2015 and April, 2016 and December, 2016 and April, 2017, according to the method described [34]. Thus, calculated as follows;
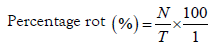
Where,
%=Percentage rotten tubers
N=Number and extent of severity of rotten tubers at the time of evaluation
T=Total number of tubers stored for the treatment
Data analysis
Data collected were subjected to Analysis of variance (ANOVA) using GenStat Discovery Edition 12 for ANOVA and mean separation. Statistical F-tests were evaluated at P ≤ 0.05. Differences among treatment means for each measured parameter were separated using Fisher’s Least Significant Difference (FLSD) [35].
Isolation and identification of pathogenic fungi from rotted yam tubers
Fungi such as A. niger, B. theobromae, and F. oxysporum, were isolated and identified from the rotted yam tubers collected.
Pathogenicity test
The pathogenicity test carried out revealed the susceptibility of healthy looking yam tubers with rot occurring more in A. niger followed by B. theobromae and least in F. oxysporum after 14 days of inoculation. The yam tubers that were not inoculated with the fungi mycelia however, did not produce any rot symptoms in the bored yam tissues throughout the period of incubation.
Effects of biological antagonist (T. harzianum) on yam tuber rot pathogens (A. niger, B. theobromae and F. oxysporum) in storage
Table 1 shows results of inoculation of healthy Ogoja white yam tubers with A. niger, B. theobromae and F. oxysporum as pathogenic fungi organisms and T. harzianum as biological antagonist in different combinations. The result showed no rot in December, 2015 in both the tubers inoculated with fungi organisms alone and in tubers paired with biological antagonist and fungi organisms. However, rots were observed in tubers inoculated with A. niger alone (11.10%) and B. theobromae alone (11.10%) in January but there was no significant difference (P ≤ 0.05) in the two treatments. In February, significant differences in rots were observed with highest percentage rot in tubers inoculated with A. niger alone (22.20%) followed by B. theobromae alone (11.10%) and F. oxysporum alone (11.10%). In March, only tubers inoculated with T. harzianum alone and F. oxysporum paired with T. harzianum showed no rot. The rest of fungi and fungi-antagonist combinations showed significant rot at varying degrees. In April, no significant differences were observed in percentage rots among the treatments. A. niger alone (44.40%) showed the highest percentage rot followed by B. theobromae alone (33.30%) compared with the least percentage rot observed in tubers inoculated with T. harzianum alone (0.00%). Mean Percentage rot after five months storage of Ogoja white yam tubers inoculated with fungi organisms and biological antagonist at different combinations revealed that the highest mean percentage rot was in tubers inoculated with A. niger alone (22.22%), followed by B. theobromae alone (15.56%), F. oxysporum alone (8.89%). The yam tubers inoculated with neither fungus nor antagonist (control) showed 6.67 % rot at the end of the five months storage period. When the same organisms were paired with biological antagonist mean percentage rots were observed to be 6.67% for A. niger with T. harzianum, 6.67% for B. theobromae with T. harzianum and 2.22% for F. oxysporum with T. harzianum respectively. This means that the mean percentage inhibitory effects of T. harzianum after five months of storage in the first year reduced rot caused by A. niger, B. theobromae and F. oxysporum in the paired treatments by 93.3%, 93.3% and 97.78% respectively while tubers inoculated with the antagonist; T. harzianum alone (0.00%) did not produce rot throughout the period of storage indicating that 100% of the tubers were protected from rot causing fungi pathogenic organisms. Storage of yam tubers between December, 2016 and April, 2017 showed no significant difference (P ≤ 0.05) in percentage rot in December, February and March but differed significantly (P ≤ 0.05) in rot caused by these pathogenic fungi in January and April. Mean percentage rot was also significant among the treatments with the highest percentage rot in tubers inoculated with A. niger alone followed by B. theobromae alone. In the second year of storage between 13.33% (F. oxysporum) and 28.89% (A. niger) of yam tubers treated with fungi alone were rotted while only between 6.67% (F. oxysporum) and 8.89% (A. niger) in the antagonist treated tubers were rotted. Tubers inoculated with neither antagonist nor fungi produced 13.33% rot (control tubers) when re-isolated and tested similar to those tubers inoculated with F. oxysporum alone. The least percentage rot was observed in tubers inoculated with T. harzianum alone (2.22%). The mean percentage rot of yam tubers for the two years. The result revealed that there was a significant difference (P ≤ 0.05) in mean percentage rot between the first year and the second year for each of the treatments probably due to differences in environmental conditions and pathogen interactions with the host yam tissue except in the treatments where B. theobromae was inoculated alone, A. niger and T. harzianum paired and B. theobromae and T. harzianum paired. The most antagonised fungus in paired treatments for both years was F. oxysporum while the least antagonised were A. niger and B. theobromae.
| Treatment | Period of storage | |||||
|---|---|---|---|---|---|---|
| Dec., 2015 | Jan., 2016 | Feb., 2016 | Mar., 2016 | Apr., 2016 | Mean | |
| 1st storage period | ||||||
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.10 ± 11.10 | 22.20 ± 11.10 | 6.67 ± 3.56 |
| A. niger alone | 0.00 ± 0.00 | 11.10 ± 11.10 | 22.20 ± 11.10 | 33.33 ± 19.20 | 44.40 ± 22.20 | 22.22 ± 7.03 |
| B.theobromae alone | 0.00 ± 0.00 | 11.10 ± 11.10 | 11.10 ± 11.10 | 22.20 ± 11.10 | 33.30 ± 19.20 | 15.56 ± 5.51 |
| F. oxysporum alone | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.10 ± 11.10 | 11.10 ± 11.10 | 22.20 ± 22.20 | 8.89 ± 5.11 |
| T.harzianum alone | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| A. niger X T.harzianum | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.10 ± 11.10 | 22.20 ± 22.20 | 6.67 ± 4.82 |
| B.theobromae X T.harzianum | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.10 ± 11.10 | 22.20 ± 22.20 | 6.67 ± 4.82 |
| F. oxysporum X T.harzianum. | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.10 ± 11.10 | 2.22 ± 2.22 |
| LSD | - | 16.66 ns | 20.40 | 33.31 | 53.97 ns | 20.53 |
| 2nd storage period | ||||||
| Dec., 2016 | Jan., 2017 | Feb., 2017 | Mar., 2017 | Apr., 2017 | Mean | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.10 ± 11.10 | 22.20 ± 11.10 | 33.33 ± 0.00 | 13.33 ± 4.36 |
| A. niger alone | 11.10 ± 11.10 | 22.20 ± 11.10 | 22.20 ± 11.10 | 33.33 ± 19.20 | 55.60 ± 11.10 | 28.89 ± 6.40 |
| B.theobromae alone | 0.00 ± 0.00 | 11.10 ± 11.10 | 11.10 ± 11.10 | 22.20 ± 11.10 | 33.30 ± 19.20 | 15.56 ± 5.51 |
| F. oxysporum alone | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.10 ± 11.10 | 22.20 ± 11.10 | 33.33 ± 0.00 | 13.33 ± 4.36 |
| T.harzianum alone | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.10 ± 11.10 | 2.22 ± 2.22 |
| A. niger X T.harzianum | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.10 ± 11.10 | 33.33 ± 0.00 | 8.89 ± 3.94 |
| B.theobromae X T.harzianum | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.10 ± 11.10 | 11.10 ± 11.10 | 22.20 ± 11.10 | 8.89 ± 3.94 |
| F. oxysporum X T.harzianum. | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.10 ± 11.10 | 22.20 ± 11.10 | 6.67 ± 3.56 |
| LSD | 11.78 ns | 16.66 | 26.33 ns | 35.33 ns | 31.16 | 18.55 |
| ns=not significant | ||||||
Table 1: Cumulative percentage rots of ogoja white yam tubers inoculated with T. harzianum (BCA) and the postharvest pathogens of white yam in different combinations.
| Treatment | Time of storage | T-value | DF | P-value | |
|---|---|---|---|---|---|
| 1st year | 2nd year | ||||
| Control | 6.67 ± 3.56 | 13.33 ± 4.36 | -5.02 | 32 | 0.00* |
| A. niger alone | 22.22 ± 7.03 | 28.89 ± 6.40 | -2.98 | 33 | 0.01* |
| B. theobromae alone | 15.56 ± 5.51 | 15.56 ± 5.51 | 0.00 | 34 | 1.00 |
| F. oxysporum alone | 8.89 ± 5.11 | 13.33 ± 4.36 | -2.80 | 33 | 0.00* |
| T. harzianum alone | 0.00 ± 0.00 | 2.22 ± 2.22 | -4.24 | 17 | 0.00* |
| A. niger X T. harzianum | 6.67 ± 4.82 | 8.89 ± 3.94 | -1.51 | 32 | 0.14 |
| B. theobromae X T. harzianum | 6.67 ± 4.82 | 8.89 ± 3.94 | -1.51 | 32 | 0.14 |
| F. oxysporum X T. harzianum. | 2.22 ± 2.22 | 6.67 ± 3.56 | -4.50 | 28 | 0.00* |
* indicates statistical significance at 0.05
Table 2: Mean percentage rot of ogoja white yam tubers inoculated with T. harzianum (bca) and the postharvest pathogens of white yam in different combinations for two years.
The results of the interactions between biological antagonist and rot fungi isolates when inoculated on healthy white yam tuber in storage showed that T. harzianum was able to inhibit the growth of A. niger, B. theobromae and F. oxysporum pathogens on Ogoja cultivar of D. rotundata and there was a significant reduction in rot caused by these pathogenic fungi on post-harvest yam tubers. T. harzianum may have acted by the production of antibiotic substances that inhibited the growth of A. niger, B. theobromae and F. oxysporum. This has been reported [19,22,36] on the production of both non- volatile antibiotics by species of Trichoderma. These substances produced by T. harzianum may be responsible in the biological control of storage rot of yam tubers, this is seen in the works of [11,16,31,33,37] where species of Trichoderma have been exploited in the control of rot fungi of tubers, fruits and vegetable diseases. The antagonistic potential of T. harzianum to inhibit the growth of the isolated fungi in storage is similar to the result of [16] who studied the biological control of rot-inducing fungi of water yam (Dioscorea alata) with Trichoderma harzianum, Pseudomonas syringae and Pseudomonas chlororaphis and found that the three antagonists significantly inhibited the growth of Botryodiplodia theobromae and Fusarium solani on yam tubers in storage [33]. Inhibited the growth of B. theobromae, A. flavus, F. solani, and Rhizopus sp. during storage of cassava roots inoculated with T. viride and recorded mean percentage rot of between 0% and 3% in the paired treatments. The use of T. harzianum in controlling postharvest fungal pathogens of yam tubers in storage for five months is similar to the work earlier on carried out [38], which used a single application of this bio-control agent and protected yam tubers in storage for up to 6 months.
Bacteria organisms have also been widely used to control fungi organisms of tuber crops [10]. In his study used Bacillus subtilis to control post-harvest fungal rot of yams in storage. In other studies, the saprophytic strain of the bacterium Pseudomonas syringe (L-59-66) also satisfactorily controlled the difficult grape rots (B. cinerea) and blue mould of citrus (P. citrinum) [39]. This saprophyte has been developed into a commercial brand (Ecosuinex). In this study T. harzianum was used to control pathogenic fungi that cause rot in yam tubers. The antagonist was able to displace the fungi organisms and inhibit their growth significantly. The study showed that there was an inhibition of the pathogenic fungi organisms when paired with the biological antagonists: T. harzianum, which may probably be attributed to the displacement of the pathogenic fungi on the Ogoja white yam tubers by causing a reduction in the percentage rot observed. This antagonist was effective in controlling rot caused by fungal organisms in yam tubers. Though A. niger and B. theobromae which resulted in biological control of the test pathogens as reported [40]. In addition, T. harzianum may also have produced antifungal substances which function by breaking down the polysaccharides, chitin, and glucans that are responsible for the rigidity of fungal cell walls, thereby destroying cell wall integrity and limiting the growth of these pathogens [41]. The result agreed with the findings [33] who recorded 0% infection in cassava tubers when T. viride was inoculated on the tubers and stored for three weeks. The control tubers that were not treated with the antagonist on the Ogoja cultivar in both years showed between 6.67% and 13.33% rot after five months of storage similar to the report [42] who reported losses due to rots in yam tubers to be 10%-15% in the first three months of storage. The results disagreed with the work [38] who estimated an average of between 20 and 39.5% of stored tubers lost to rot organisms while [43,44] also reported that 50% of the yams tubers produced and harvested in Nigeria are lost to diseases in storage. The result showed that the biological control agent was able to reduce rot more in the first year compared with the second year. This could probably be due to favorable environmental condition which increased the interaction of pathogens with the host yam tissues and decreased the potentials of T. harzianum. Similarly, it has been reported that fungal species occurred more abundantly in the more humid months where the environmental conditions favored the production of inoculum more than in the drier less humid period [45].
The finding has revealed that T. harzianum has potentials to control rot causing pathogens in post-harvest yam cultivars. This can complement or provide better alternative ways of reducing rot in yam tubers than in the use of chemical fungicides which are often very expensive and environmentally hazardous. There is an increased interest in the reduction of synthetic chemical residues and prevention of resistance development through utilization of biological products particularly from Trichoderma spp. which are believed to present the highest potential as a commercial bio fungicides around the world [20,46,47].
The study has demonstrated that T. harzianum has the potential to control postharvest rot fungi pathogens of yam tubers in storage over a long period of time. It has also been shown that the antagonist was more effective in reducing rot caused by F. oxysporum compared with A. niger and B. theobromae in both years of studies. The biological control agent could therefore, be considered as a substitute to synthetic fungicides in managing postharvest tuber rots of yams since it is eco-friendly, cheap and biodegradable.
The authors declare that there is no conflict of interest regarding the publication of this paper.
This research received no specific grant from any funding agency in the public, commercial or not-for- profit sectors.
Citation: Gwa VI, Ekefan E (2021). Potential for Biological Control of Postharvest Fungal Rot of White Yam (Dioscorea rotundata Poir) Tubers in Storage with Trichoderma harzianum. Virol Mycol. 10:210.
Received: 05-Apr-2021 Accepted: 19-Apr-2021 Published: 26-Apr-2021 , DOI: 10.35248/2161-0517.21.10.210
Copyright: © 2021 Gwa VI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.