Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2022)Volume 13, Issue 5
Objective: Parthenium hysterophorus L. extract was employed to ascertain the antimicrobial attributes in combination with standard antibiotics and its biofilm inhibitory effects against some selected pathogens.
Methods: The ethanolic extract was tested for antibacterial susceptibility against gram positive and gram-negative bacteria by employing agar well diffusion method for Minimum Inhibitory Concentrations (MIC) and Minimum Bactericidal Concentrations (MBC). The synergistic effect of combined plant extract and antibiotics was assessed using time-kill assay and checkerboard methods. Biofilm inhibition assay was also carried out to test inhibitory effect of plant extract.
Results: The plant extract was extracted to have important phytochemicals and it showed antibacterial activity against all the selected pathogen as on par with standard antibiotics, as maximum of 22 mm diameter of zone of inhibition was observed against B. subtilis; the optimal MIC and MBC was obtained and its index was also measured, with 0.156 mg/ml the extract showed the maximum inhibitory activity against B. subtilis; the biofilm of gram negative bacteria was inhibited by the plant extract marginally higher than the biofilm formed by gram positive bacteria; the time kill assay showed (-2.5 log10 CFU/mL) decrease in cell density by the two times combined effect of extract and antibiotic. Positively agreeing that, one hundred percent of interactions were occurred with B. subtilis. The output comparison between checkerboard and time-kill assays showed the level of agreement is 100% for B. subtilis, the partial agreement was presumed to be with E. coli whereas, others showed a good agreement. Synergisms using the time-kill constituted about 100% in B. subtilis and S. aureus, and K. pneumoniae, while indifference was occurred in E. coli when compared with checkerboard assay.
Conclusion: The assessment proved that P. hysterophorus extract was shown to be having broad spectrum antimicrobial compounds. Hence, further study is indispensable to isolate bioactive pure compounds.
Antibacterial activity; Zone of inhibition; Synergism; Antibiotics; Time-kill assay; Biofilm
Infectious diseases are a prime concern mainly in third-world countries where a significant illness and deaths occurred for humans. Bacteria in general are having inherent capacity, to be utilized genetically, to acquire and bestow resistance to drugs hence being used as therapeutic agents [1,2]. Hence, public health is at stake due to multidrug resistant profile pertaining newly mutating bacterial strains or opportunistic bacterial species have been challenging for academic and pharmaceutical industries for their incidences are undeniably involved mammoth efforts to solve it [3].
Moreover, biofilm, a complex matrix of microbial communities of microorganisms, is composed of poly saccharides, proteins adhered to biotic or abiotic substrates. This enabled them to resist any harsh conditions both environmentally and human induced antimicrobial actions. Moreover, such mutated form microbes tend to transmit their resistant strain to other bacteria. Especially, those immuno-compromised candidates seemed to have severe clinical problems such as diarrhoea causing serotypes of E. coli as well as implicated for their food-borne disease across the various parts of the world. Therefore, finding an appropriate drug for the effective antimicrobial treatment are perpetually recapitulated [4-6].
Previously, the ideal administration of plant extract alone showed efficacy against the pathogen microbes, this is however does not guarantee in all the instances ensues simultaneous management of two or more drugs along with plant extracts to achieve the expected outcome of treating not only free-living pathogen also in co-existing pathogens as well [1,7]. For instance, beta lactams and aminoglycosides containing bacteria are approved to be treated with vancomycin, chloramphenicol etc. are reportedly resistant. And, fluoroquinolone antibiotics, plasmid mediated quinolone resistance (qnrA gene) in E. Coli and members of Enterobacteriaceae are reportedly showing low to high level resistance against ciprofloxacin and other fluoroquinolones antibiotics.
Having understood the efficacy of plant extract alone in various other studies against pathogenic microbes, biologically active agent now being in combinations is addressed by the scientific forum for almost a decade. Moreover, the combination model of treatment has been reported to be effective against drug resistant microbes, to reduce toxicity hence, augmentation in clinical methodology by incorporating synergistic antimicrobial activity. Plant extract-based bio compounds have shown a promising effect upon inhibition of MDR-efflux pump, and beta lactamase activity, and R plasmid elimination which are presumably conferring property of antibiotic resistance [1,8].
However, a long-requiring trail must be in place in order to understand the etiological attributes of different relationships among pathogens, and susceptibility for mutation as far as synergistic combinations of drug before being implemented. Taken the aforementioned objectives, the combination potential of extract of P. hysterophorus with antibiotics, biofilm inhibition activity, was assed.
Plant collection
P. hysterophorus was collected manually from in and around the vicinity part of Hindusthan College of Arts and Science, Coimbatore, Tamilnadu, India. The collected plant material was washed with running tap water for 2-3 times followed by sterile water wash and then allowed to shade dry for 24 hours after cutting into small pieces. The plant material now was powdered and stored for further analysis.
Pathogens employed for this study
Gram negative bacteria such as, Escherichia coli, Klebsiella pneumoniae, Bacillus subtilis and Staphylococcus aureus of gram- positive bacteria were served as test organisms in this study.
Preparation of plant extracts
100 grams of dried plant material was weighed with 500 ml of ethanol, which acts as extractant, under seldom shaking at room temperature, which is around 25 ± 2°C, for 24 hours. With triplicate measurements, the obtained crude extract was filtered using Whatmann filter paper No 42. The rotary evaporator helped to concentrate the crude extract in order to dry under a reduced pressure in place at 45°C beside of preparing ethanol stock solution for 200 mg/ml, and stored at 4°C in dark room for future use [8,9].
Qualitative phytochemical screening
Phytochemical screening was carried by using a standard method which follows; Mayer’s, Wagner’s, Hager’s, Dragendorff’s methods for alkaloids, Molish’s, Fehling’s, Barfoed’s, Benedict’s methods for carbohydrates, Foam test for saponins, Borntrager’s, Legeal’s methods for glycosides, Million’s, Ninhydrin, Hopkins- Cole tests for proteins and amino acids, Libermann-Buchard’s method for phytosterols, Noller’s test for terpenoids, alkaline, magnesium and hydrochloric acid reduction tests for flavonoids, ferric chloride test, gelatin test, lead acetates tests for phenolic compounds, neutral Fecl3 for tannins, and using absolute alcohol gum and mucilage was detected [9].
Quantitative determination
Total flavonoid content: A spectrophotometric-aluminium chloride method was employed to ascertain flavonoids. A 0.2 ml of extract, which is 2 mg/ml, was taken in DMSO along with 1.8 ml of methanol, 0.1 ml of 1 M potassium acetate, 0.1 ml of 10 percent aluminium chloride and 2.8 ml of distilled water. Following the incubation at room temperature for about 30 minutes then the absorbance of mixture containing extract was measured at the wavelength of 415 nm. The quercetin standard solution was prepared for 1 mg/ml in ethanol to calibrate the curve. Different volumes, such as 20-200 mg of quercetin together with 1.8 ml of ethanol, 0.2 ml DMSO was also prepared. A triplicate measurement was performed for all the experiment, the data obtained was expressed as mean ± SEM. The flavonoid quantity was measured with an expression of mg QE per mg [1,4].
Phosphomolybdate assay: Total antioxidant activity of the plant extract was performed using phosphomolybdate method. The standard, propyl gallate, was also prepared in order to calibrate the data. One hundred micro grams of the extract solution was prepared in DMSO, along with 0.25 mL ethanol, 3 mL of reagent containing 0.6 M sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate to represent 0.05 ml. The reaction mixtures were incubated at 95°C for 90 minutes or till it appears to have green colour to carry out measurements of absorbance at 695 nm wavelength spectrophotometrically. The total antioxidant capacity was expressed as μg propyl gallate equivalents per gram of sample (μg PGE/mg of sample) by using the standard graph. The results were expressed as mean ± SD (n=3) [1,4].
Zone of inhibition assay by agar diffusion
The determination of antibacterial activity of the ethanolic extract in comparison with standard antibiotics for gram positive, ciprofloxacin was used whereas, for gram negative bacteria which is penicillin was performed by employing modified Kirby-Bauer diffusion technique, involving saline suspension of each adjusted bacterial isolates was swapped with Mueller-Hinton agar plates. The wells were formed using a heat sterilized 6mm cork borer in the agar medium subsequently, filled with 100 μl of the following concentrations i.e., 0.25 mg/mL, 0.5 mg/mL, 1 mg/mL, 2 mg/ mL, 2.5 mg/mL, 5 mg/mL and 1 mg/mL of standard antibiotics having taken good care of ensuring no spillage onto the agar surface or any experimental set up. Given the incubation time of one hour to make sure the proper diffusion of the solution further, incubated at 37°C for 24 hours followed. A negative control was maintained with 10% DMSO in test plates without extract. The test batch was carried out in duplicate. Following 24 hours of incubation, the plates were examined for zone of inhibition if any; subsequently diameter of the same was also measured in millimeters.
An agar dilution method was performed to understand the antibacterial activity of the extract with different concentrations of extract ranging from 100 to 10000 μg/ml in Mueller-Hinton agar. The standardised given bacterial cultures were dissipated onto the agar medium aseptically. The negative control, 5% ethanol, without extract was maintained. Having a triplicate in operation, the plates were incubated at 37°C for 24 hours. The plates of different concentrations observed to have no visible growth of the organisms were treated as minimum inhibitory concentration [1,2,7].
Minimum inhibitory concentration determination
The viability of pathogenic organisms can be ascertained by defining the lowest concentration of extract at which the reduction of cell viability was established, using serial tube dilution technique. At 0.0195-5 mg/mL of crude extract and 0.016-1 mg/ mL of ciprofloxacin or penicillin of various concentrations were prepared in the Mueller Hinton broth medium. With 100 μl of standardized bacterial culture being inoculated, two blank broth tubes; one is with inoculation and another without inoculation were set up to assess the growth and sterility. After the incubation at 37°C for 24 hours, the tubes were observed in order for the extract/antibiotics to manifest their minimum inhibitory effect by assessing the first tube in the series of tubes of different concentrations at which there was no dissemble growth, to be reckoned as Minimum Inhibitory Concentration (MIC) of extract/antibiotics against the tested pathogens [9].
Minimum bactericidal concentrations determination
Among the series of concentration of extracts/antibiotics, the first tube which is macroscopically discernible was picked up to ascertain Minimum Bactericidal Concentration (MBC). The tube containing mixture was gently stirred it using a sterile pipette soon after that, sampling quantity of 100 μL aliquot was removed to place onto an antibiotic free nutrient agar using a single streak method and allowed to stand for a while for impregnating the medium. The method of sub-culturing was carried out in order to eliminate the antimicrobial agent carry-over from the 100 μL sub cultured volume besides being maintained for growth and sterility control. The plates were incubated for 24 h at 37°C later to that, found the Minimum Bactericidal Concentration (MBC) over the tubes containing lowest concentrations that did not produce any bacterial growth was regarded as MBC values of the extract/antibiotics. The observed value was compared with value of MIC that did not portray the evidence of growth of pathogen following 48 hours of incubation [9].
Screening for the potential of anti-biofilm
Prevention of initial bacterial cell attachment: The effect of extract upon prevention of cell attachment was assessed using biofilm inhibition assay. The standardised culture, which at OD560 equals to 0.02 nm of wavelength translating to 1.0 × 106 CFU/mL of E. coli, K. pneumoniae, and B. subtilis, S. aureus was inoculated into the 96 well microtiter plate and kept at 37°C for 4 hrs without shaking. A 100 μl of aliquot of plant extract, which is corresponding to 2 mg/ml, was added along with triplicates subsequently; the titre plates were brought it to the final concentration of 1 mg/ml and kept for incubation 37°C for 24 hours. Having ethanol served negative controls and antibiotics only containing positive control, the biomass was quantified employing the modified crystal violet staining method [1,7,10].
Anti-biofilm activity against established/performed biofilm: The plant extract anti-biofilm potential was examined against the established or preformed biofilm. A standardised bacterial culture at OD560 equals to absorbance of 0.02 nm which further translated to 1.0 × 106 CFU/ml of E. coli, K. pneumoniae, and B. subtitlis, S. aureus was inoculated into the 96 well microtiter plate and incubated at 37°C for 24 hours, for their microbial irreversible attachment phase, or 48 hours; for their mature biofilm formation, without shaking so as to enable bacteria to form multi-layered biofilm. While ethanol served as negative control, antibiotics were used alone as positive control. The developed biomass further analysed employing modified crystal violet staining assay.
Crystal violet staining assay: The 96 well micro titre plate was washed five times with sterile distilled water and air dried further, oven-dried at 60°C for 45 min. the well containing biofilm was stained with 100 μl of 1% crystal violet and kept at room temperature for 15 minutes then the plate was washed with distilled water to wipe off any unabsorbed stain. Biofilm now perceived as purple rings on the side of the well [2]. The semi- quantitative assessment of biofilm-formation was carried out using a 125 μl of ethanol to destain the wells. Having removed 100 μl of aliquot from the destained well, the absorbance of the sample was measured thus its percentage of inhibition of biofilm was determined using the equation given below:
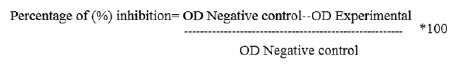
Synergistic effect of extract and antibiotic assay
Time-kill assay: The 10 ml of Mueller Hinton broth at 1/4th of MIC, and two times of MIC were taken in McCartney bottles. Two controls, which is the medium with only test organism, and with only extract, were maintained. A 10 ml of inoculum in terms of 105 CFU/mL cell density was added in the McCartney bottles and control bottles. Following the incubation at 37°C on orbital shaker at 120 rpm, 0.5 ml of each suspension was withdrawn at the appropriate time interval and transferred to tube containing 4.5 ml nutrient broth recovery medium; includes 3% of tween-80 in such a way as to neutralize the effects antimicrobial properties from the test suspensions.
The suspension was serially diluted till 105 dilution using sterile buffered physiological saline. Added a 0.5 ml of final dilution in to the pre-sterile nutrient agar maintained at 45°C thus plated out. The plates were then incubated at 37°C for 24 hours besides being maintained with control setup, which carried with no extract or antibiotic. After ensuring viability on every sample, number of colonies were expressed as Log10: 2 Log10 defined to be a Synergy (S), which implies a decrease in number of colonies at 24 hours by the combination compared to the most active single performer, whereas ≥ 2 Log10 CFU/mL stands for viable and surviving organisms in the active combinations at the outset of inoculum, while <2 Log10 describes the Indifference (I) of colony counts which increased at 24 hours with combination compared by most active singer performer, and ≥ 2 Log10 defines an increase in colony count at 24 hours by the combination compared with that by the most active single agent alone [1,5,8].
Checkerboard assay: A standardised culture was inoculated into the plates following streaking with duplicates and incubated at 37°C for 24 hours. Having observed the MIC values, the Fractional Inhibitory Concentration (FIC) was thus inferred from lest concentration of extract and antibiotic combinations ensuing no discernible growth of the test organisms. The FIC values can be ascertained using the standard formula;



The Factional Inhibitory Concentration (FIC) index was thus evaluated following this, where FIC is <1 stands for Synergy; when equivalent to 1, implies Addition; if >1, describes to be Indifference; and finally, if ≤ 2, then it indicates Antagonistic. When there was a change in the MIC value resulting from more than one combination of the antibiotic or extract, the FIC value was communicated as the average of the single FIC values [5].
Statistical analysis
One-way ANOVA was employed to determine the antibacterial activity of plant ethanolic extract while compared to standard respective antibiotics [8]. Thus, obtained values were expressed as mean ± SD using SPSS version 16 software. The independent mean value of Checkerboard and time-kill assay was compared using unpaired t-test, resulting P-values <0.05 were considered statistically significant (Tables 1-6).
| S. No | Phytochemicals | Ethanolic extract |
|---|---|---|
| 1 | Alkaloids | + |
| 2 | Carbohydrates | + |
| 3 | Saponins | + |
| 4 | Glycosides | + |
| 5 | Proteins and amino acids | + |
| 6 | Phytosterol | + |
| 7 | Phenolic compounds | + |
| 8 | Flavonoids | + |
| 9 | Terpenoids | + |
| 10 | Tannins | - |
| 11 | Gum and mucilage | + |
| 12 | Flavonoid | 17.6 ± 0.21 QE/mg* |
| 13 | Total antioxidant activity (Phosphomolybdate assay) | 190 ± 0.13* μg PGE/mg |
Note: *The result is expressed as mean ± SD (n=3).
Table 1: Qualitative and quantitative phytochemical analysis of P. hysterophorus.
| Tested bacterial isolates | Ciprofloxacin/Penicillin | Average zones of inhibition as a function of mg/mL | ||||||
|---|---|---|---|---|---|---|---|---|
| Agar diffusion assay | Agar dilution | |||||||
| 1 | 0.25 | 0.5 | 1 | 2 | 2.5 | 5 | MIC | |
| E. coli | 26 ± 0.2 | 0 ± 0.0 | 00 ± 0.1 | 06 ± 0.1 | 11 ± 0.2 | 14 ± 0.2 | 16 ± 0.2 | ≤ 0.5 |
| K. pneumoniae | 23 ± 0.3 | 7 ± 0.2 | 15 ± 0.2 | 16 ± 0.2 | 18 ± 0.1 | 19 ± 0.4 | 20 ± 0.3 | ≤ 0.2 |
| B. subtilis | 25 ± 0.4 | 8 ± 0.4 | 15 ± 0.3 | 16 ± 0.0 | 17 ± 0.2 | 18 ± 0.1 | 22 ± 0.2 | ≤ 0.1 |
| S. aureus | 19 ± 0.2 | 4 ± 0.2 | 13 ± 0.2 | 15 ± 0.2 | 16 ± 0.3 | 17 ± 0.2 | 19 ± 0.1 | ≤ 0.2 |
Table 2: Results of agar diffusion assays to determine the antibacterial activity of the crude ethanolic extract of P. hysterophorus extract.
| Tested bacterial isolates | Macro broth dilution | |||||
|---|---|---|---|---|---|---|
| Ciprofloxacin/ Penicillin (mg/mL) | Ethanolic extract (mg/mL) | |||||
| MIC | MBC | MICindex | MIC | MBC | MICindex | |
| E. coli | 0.019 | 0.039 | 2 | 0.625 | 1.25 | 2 |
| K. pneumoniae | 0.156 | 0.156 | 1 | 0.312 | 0.312 | 1 |
| B. subtilis | 0.039 | 0.078 | 2 | 0.156 | 0.156 | 1 |
| S. aureus | 0.312 | 1.25 | 4 | 0.312 | 0.312 | 1 |
Table 3: Antibacterial activity of the ethanolic extract of P. hysterophorus extract.
| Extract/antibiotics | Biofilm inhibition expressed in percentage (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. Coli | K. pneumoniae | B. subtilis | S. aureus | |||||||||
| 4th hr | 24th hr | 48th hr | 4th hr | 24th hr | 48th hr | 4th hr | 24th hr | 48th hr | 4th hr | 24th hr | 48th hr | |
| P. hysterophorus | 84 | -25 | 20 | 80 | -35 | 10 | 74 | -10 | 40 | 80 | -30 | 10 |
| Ciprofloxacin/ penicillin | 95 | 80 | 90 | 85 | 80 | 92 | 93 | 88 | 110 | 85 | 80 | 95 |
Note: The numbers >0% ≥ 50% is considerably low activity, >50% (in bold) show high activity against the bacteria. Negative values denote enhancement of biofilm.
Table 4: Effect of ethanol crude extract of the plant against biofilm formation by gram-negative and gram-positive bacteria after 4th, 24th and 48th hours.
| Bacterial isolates | Synergy | Synergistic effect ( ± 0.1 CFU/ml) | |||
|---|---|---|---|---|---|
| E. coli | K. pneumoniae | B. subtilis | S. aureus | ||
| Extract and ciprofloxacin/ penicillin | ¼ MIC | -1.4 | -1.2 | -2.1 | -1.4 |
| 2 MIC | -2.5 | -2.3 | -2.3 | -2.4 | |
Note: Numbers of colonies were counted and expressed as log10; expressed as mean ± SEM (n=3).
Table 5: Anti-bacterial activity of standard antibiotic plus extracts combinations using time-kill assay after 24th hours.
| Bacterial isolates | Checkerboard (FIC index) | |||
|---|---|---|---|---|
| E. coli | K. pneumoniae | B. subtilis | S. aureus | |
| Extract + ciprofloxacin/ penicillin | 1.4 (I) | 0.6 (S) | 0.4 (S) | 0.8 (S) |
Note: I: Indifferent; S: Synergy
Table 6: Anti-bacterial activity of standard antibiotic plus extract combinations by checkerboard method.
Phytohormone assessment
The present study revealed that the ethanolic extract of the plant contained alkaloids, carbohydrates, saponin, glycosides, flavonoids, glycosides, protein and amino acids, phytosterol, phenolic compounds, flavonoids, terpenoids, gum and mucilage with one exception tannins was not present in the plant extract. The flavonoid 17.6 mg and Antioxidant activity 190 ± 0.13* μg PGE/mg was observed in Table 1.
Antibacterial activity and zone of inhibition
The diameter of zone of inhibition of ethanolic extract of the plant in different concentrations, in comparison with standard antibiotics; which is penicillin for gram negative and ciprofloxacin for gram positive bacteria is tabulated in Table 2 besides showing agar dilution assay to interpret the antibacterial activity. The zone ranged from 0 mm to 16 mm was observed in E. coli containing plates, 7 mm to 20 mm diameter in K. pneumoniae of gram-negative bacteria, while the standard antibiotic, penicillin was shown to have 26 mm and 23 mm of diameter in E. coli and K. pneumoniae containing plates respectively. Whereas, zone of inhibition in gram-positive bacteria containing plates i.e. B. subtilis was noted to have 8 mm to 22 mm diameter, while in S. aureus plates, ranged from 4 mm to 19 mm, as concurrently compared with an effect of standard antibiotic ciprofloxacin, showed 25 mm and 19 mm of diameter in B. subtilis and S. aureus containing plates respectively. The findings showed that the crude extract of plant unveiled antibacterial actions against almost all the test gram-positive and gram-negative bacteria when assessed at a concentration beyond 1 mg/ml till 5 mg/mL. While assessing the reproducibility of the data obtained was deemed to have good agreement between 0.1 mm to 0.4 mm in all cases, considering the heterogenous nature of extract, this much of reproducibility is very promising as shown in Table 2.
MIC and MBC
The plant extract and antibiotics exhibited MIC and MBC against the test organisms is tabulated in Table 3 .The ethanolic extract showed a varied MIC between 0.312 mg/mL and 0.625 mg/mL against the selected pathogens. The MIC of the plant extract in gram negative bacteria that is E. coli containing plates was 0.625 mg/mL; 0.312 mg/mL in K. pneumoniae, while the corresponding antibiotic, penicillin showed 0.19 mg/ml and 0.156 mg/ml respectively; whereas, the gram positive bacteria i.e. B. subtilis was inhibited at 0.156 mg/mL, and 0.312 mg/ml in S. aureus containing plates while, the corresponding antibiotic ciprofloxacin showed 0.039 mg/ml, 0.312 mg/ml respectively. The Minimum Bactericidal Concentrations (MBCs) was also ascertained which ranged from 0.156 mg/mL to 1.250 mg/mL.
The index of MIC of extract and antibiotics was interpreted using MBC is also presented in Table 3. The extract showed a maximum index of two (2) against E. coli, whereas in all other circumstances, the index was defined to be one. The index of penicillin was measured to be two and one with E. coli and K. pneumoniae respectively, while the index of ciprofloxacin was two and four with B. subtilis and S. aureus respectively.
Biofilm inhibition
Inhibition of development of few hours (4 hours) incubated biofilms in terms of assessment of destruction, and the potential of anti-biofilm properties of the plant extract against the pre- formed biofilm after 24 or 48 hours was further established is presented in Table 4.
An established criterion, which is percentage, was applied to find out the degree of inhibition. If the percentage inhibition values in between 0% to 100%, signifies inhibition of biofilm, while enhancement of growth is reflected with values if it is less than 0%. The 50% of inhibition implies the effect of extract used to be good, while it is poor with 0% to 49%. The plant extracts showed varying degrees of impact on the prevention of attachment. The extract explored good prevention of biofilm attachment in all cases, that is above 80% at 4 hours development of biofilm, whereas, following 24 hours development showed an enhancement of bacterial growth in both gram positive and negative bacteria that is between (-10%) to (-35%) invariably in all cases, translates to be not inhibiting the growth of bacteria especially, the extract showed the poor inhibition of (-35%) against the attachment of K. pneumoniae.
However, at 48th hour post development stage, the effect of extract over the bacterial biofilm inhibition was shown between 10% to 40%, as maximum of B. subtilis at 40% of inhibition. On the other hand, when compared to standard antibiotics at 4th and 48th hour post development inhibition was marginally higher in all cases. At 24th or 48th hours post development stage the extract gave a significant percentage of inhibition. When compared, the gram-negative bacteria were shown to have a marginal resistance to the plant extract than gram-positive bacteria especially E. coli. The properties of extract examined against the pathogens mitigated the attachment of the bacteria as reflected from their respective MIC’s and MBC in Table 2.
Time-kill assay (synergy)
The effect and synergic interactions between antibiotic plus extract combinations in terms of time-kill assay is presented in Table 5. The combined effect of both extract and antibiotic have already explored for their efficacy separately for their bactericidal effect against both selected gram-negative and gram-positive pathogens.
The results presented by means of changes in log10 of viable colonies inferred that the extract exhibited a significant bactericidal activity. After 24 hours incubation, the bacteria with extract and antibiotic combinations of 1/4th of MIC and 2 times of MIC, the log decline in the viable colony count from gram negative bacteria is (-1.4 CFU/ml) and (-2.5 CFU/ml) in E. coli, (-1.2 and -2.3 CFU/ml) in K. pneumoniae, whereas from gram positive bacteria, it was (-2.1 and -2.3 CFU/ml) in B. subtilis and (-1.4 and -2.4 CFU/ml) in S. aureus respectively recorded. Among the pathogens, between 1/4th and 2 times of MIC, the cell density decline of B. subtilis was observed to be marginal with that of others which showed significant difference.
As far as the synergistic combination of 2 times of MIC is concerned, with above 2 log10 observed in all cases translating the most viable cell reduction, while in 1/4th of MIC showed to be indifference which is <2 log10; which is corresponding to be moderate viable cell reduction. Nevertheless, in both circumstances, the cell viability of B. subtilis recorded with above 2 log10 reduction. The experimental results are positively agreeing that, one hundred percent of interactions were occurred with B. subtilis, while insignificant interactions of 50% presumed to be formed in all the other bacteria between 1/4th and 2 times of MIC of combinations of extract and antibiotics in Table 5. The reproducibility rate of the combined drug effect upon the bacteria showed 0.1 CFU per ml observed to be good as the environment factors might cause interferences in the experiment stability.
Checkerboard assay
Anti-bacterial activity of standard antibiotic plus extract combinations by checkerboard method is presented in Table 6. The Fractional Inhibitory Concentration (FIC) index was obtained to be <1 was considered synergy in K. pneumoniae (0.6) of gram-negative bacteria and S. aureus (0.8) and B. subtilis (0.4) of gram-positive bacteria, whereas, the synergic index of FIC was >1 was defined to be indifference in E.coli (1.4) of gram negative bacteria. The output comparison between checkerboard and time-kill assays in both 1/4th and 2 times of MIC synergy, showed the level of agreement is 100% for B. subtilis; the most susceptible organism against the synergic combination of the extract followed by susceptibility of K. pneumoniae and S. aureus showed good with 2 times of MIC’s synergic combination, the partial agreement was noted in the case of E. coli.
The secondary metabolites of P. hysterophorus are reported to contain many biologically active and therapeutic properties, which are in other words, do have potency of medicinal purposes. The extraction using ethanol was showed to be high in yield as compared to other extractants; which could be caused by the polarity of the solvent, can pull off as much as constituents from the extract. It is evident that, presence of rich in content of flavonoids which has been assessed as the most significance bioactive compounds upon scavenging activity. As far as bioprospecting, yield of bio-compounds enlightens the scope of antibacterial activity; having explored that, gram negative bacteria showed resistance than gram positive. This is attributed that morphological feature of cell walls of gram- negative bacteria contains hydrophilic lipopolysaccharide based outer layer, indulged to show resistant to the imbibing effects of antibacterial agents beside of having some enzymes in and around the periplasmic space which is able to break down antibacterial compounds [4].
Alkaloids is reported to have antimalarial, antiasthma, anticancer, cholinomimetic, vasodilatory, antiarrhythmic, analgesic, antibacterial effects; carbohydrate-based compounds are broadly used in therapeutics for cardiovascular and haematological treatments such as, inflammatory and anti-thrombotic treatments. Saponins showcase potential against bacteria, fungi and viruses apart from their indispensable action on stimulate the yield of T-cells as well as acting as antioxidants and scavenging oxidative stress. Glycosides play a vital role for treating heart failure. Plant sterols tend to lower cholesterol levels and aid prevents heart disease employed in some cancers and weight loss. Phenolic components are reported upon antioxidants, anticancer, antibacterial activities. Flavonoids can strengthen blood vessels, while naturally occurring terpenoids are hydrocarbons used in antihyperglycemic, anti-inflammatory, antioxidants, antiparasitic, immunomodulatory, and as skin permeation enhancer. Tannin used to treat tonsillitis, pharyngitis, hemorrhoids, and skin eruptions. Gums and mucilage’s used in therapeutics for their demulcent properties for cough suppression, relieves irritation of mucous membranes, thickening agents, emulsifiers, and stabilizers in food industry, and ingredients of dental and other adhesives as well as bulk laxatives [4].
The index of MIC and MBC was shown to have varied in both antibiotics against all the selected pathogen, it might be due to unstable nature of commercial antibiotics, and shelf life integration was drastically influenced by the environmental factors. Biofilm generated by bacteria has shown an aggravative nosocomial infections, thus the priority goes for finding environmental and economic friendly drug choice. This experiment has shown a promising effect on the destruction and reducing colonization of bacteria. The extract has precisely interfered, in the formation of biofilm as has been test during 4th and 24th and 48th hours against the four pathogens, with Brownian, sedimentation, Lifshitx-Van der Walls and electrostatic interaction-based forces; which inclined to favour adherence of pathogen on to surface. Apart from halting the availability of nutrients by blocking receptors channels in the port system might have triggered for the disintegration of from the substratum, thus preventing infections. The results are substantiated further by the report configured with ethanolic crude extract of P. guajava (Myrtaceae) to mitigate the attachment of S. mutans, a familiar pathogen to form biofilm upon oral surfaces [2,8].
Barring the degradative effect upon biofilm formed after 24 hours, the plant extract showed an excellent performance against 48 hours with all the selected pathogens. It was believed that, a preformed biofilm is not easy to eradicate with a support of some reports saying that, pathogens-based biofilm could resist antimicrobial properties and still exhibit infections upon biotic and abiotic surfaces. The underlying causes were the presence of extracellular polymetric matric stimulates firm attachment which ensures low penetration of antibiotics. However, on the contrary to that belief, extract could discommode the cell to cell communication (quorum sensing) of bacteria, hence biofilm reduction. The study strongly enlightens the effectiveness of biofilm inhibition in various stages which must be regarded in clinical applications which can be an immunological defense by the infected host. Hence, elucidate further to comprehend the molecular bases of anti-biofilm interaction might help us to understand better [7,9,10].
Mutual cooperation in terms of combined effects of both antibiotic and extract has been confirmed and substantiated with the various other reports however; the use parthenium is presumably a first-of-its-kind study in synergistic drug effect. Already established drug potential of parthenium is further improved by the results obtained which in support of no antagonism reported. There was also good agreement between the combinations using time-kill assay and checker board method was to precise perfection of 100%. The combination under time kill study was using 1/4th and 2 times of MIC showed degree of inhibition was improving or constantly persisting even after 24 hours incubation in support of principles of synergistic effect [9].
The antibiotic explored synergism with the plant extract against all tested bacteria, though with degree of antimicrobial activity profiles with regard to the concentrations. However, the findings suggested the potential utilization of extract of the plant and a standard antibiotic as a synergistic therapy towards the cure of infections caused by E. coli, K. pneumoniae, B. subtilis and S. aureus. The findings can therefore be advocated further, due to their medical suitability in terms of antimicrobial synergistic effects, upon in vivo study to inspect the claim [2,3,7].
The study portrayed a few new things that never been explored before, the parthenium extract in synergistic effect with antibiotics was investigated to show significant combination. Being toxic in nature and invasive attributes, the plant extract has shown to have potential phytogenic compounds and their promising effects on pathogens. The phytochemical has proved for its potency against pathogens when it is stand alone or together as on par with standard antibiotics. The claim can further be examined to determine their applicability in clinical conditions.
[Crossref] [Google Scholar] [PubMed]
[Crossref]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Godson ASG (2022) Phytochemical and In vitro Assessment of Synergistic and Biofilm Inhibitory Effects of Parthenium hysterophorus L. J Agri Sci Food Res. 13:502.
Received: 05-Sep-2022, Manuscript No. JBFBP-21-007-Pre-QC-22; Editor assigned: 07-Sep-2022, Pre QC No. JBFBP-21-007-Pre-QC-22 (PQ); Reviewed: 22-Sep-2022, QC No. JBFBP-21-007-Pre-QC-22; Revised: 27-Sep-2022, Manuscript No. JBFBP-21-007-Pre-QC-22 (R); Published: 06-Oct-2022 , DOI: 10.3389/2593-9173.22.13.502
Copyright: © 2022 Godson ASG. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.