Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2019)Volume 8, Issue 2
Eucalyptus is expressed as huge potentials for Ethiopian climatic conditions. Looks for quality of essential oils was an aim for this study. Hydro distillations of fresh leaves were yielded 1.75% (E. globulus) and 2.00% (E. citriodora) essential oils. Major compounds with 1, 8-Cineole (77.24%) and α-Pinene (10.54%) were indentified from E. globules essential oil. Eucalyptus citriodora essential oil was abundant with Citronellal (73.86%) and Citronellol (14.13%). The results of DPPH assay indicated that weak free radical scavenging activities with IC50 value of 795.42 mg/mL for E. globulus, 169.64 mg/mL for E. citriodora and 0.577 mg/mL for the reference Ascorbic acid. The overall quality of E. globules and E. citriodora essential oils are comply the international specifications.
Antioxidant; Essential oil; Ethiopian; Eucalyptus citriodora; Eucalyptus globulus
Eucalyptus is the genus native to Australia with family Myrtaceae, comprises over 700 species [1] and cultivated worldwide as a purpose of fuel wood, timber, pulp, essential oil, gum, medicine and aesthetic value. The abundance of Eucalyptus globulus Labill (Blue gum or Tasmanian blue gum) in Ethiopia is linked to the establishment of Addis Ababa city and occurrence of fuel wood shortage in the year 1894-1895. Therefore plantation of Eucalyptus was taken as a solution by initiating the idea of two French key persons, Mondon-Vidaillet and O'Brien who were proposed to emperor Menilik II. Recently it is known as "Nech bahirzaf " and expected to established plantation over 100,000 hectares [2]. On the other hand, Eucalyptus citriodora (Lemon scented gum) had expected to be established at Wendo Genet Agricultural Research Center before five decade years by Belgium investors for perfumery essential oil production. Now locally known as "Yeshito Zaf " and covered over 50 hectares land.
According to Dagne et al. [3] report Eucalyptus oils are generally classified in to three types; Cineolic, perfumery and industrial. Eucalyptus globulus essential oil is rich in 1, 8-cineole which has the range 54-95% [4]. Non-clinical and clinical data on 1,8-cineole support that an effect of Eucalyptus oil on upper respiratory diseases and in muscular pain is plausible [5]. Eucalyptus citriodora essential oil is the world larger volume traded perfumery oil [6] and Citronellal is the majorly abundant compound.
Eucalyptus oil content is known to vary depending on geographic location and time of harvest [7]. Besides yields, the important concepts related to quality of essential oils are the chemical composition and its biological activities. Therefore, this work had designed to define the quality and antioxidant activity of two Eucalyptus species essential oils obtained from potential areas of Ethiopia.
Sample collection
Leaf samples of E. globulus were collected from 5 meters height tree at Kofele, Arsi Zone (2689 m a.s.l., N 07°05' 575", E 038° 47' 705"). Leaf of E. citriodora was harvested from 3.5 meters height tree at Wendo Genet Agricultural Research Center compound (1800 m a.s.l., N 07° 05' 576" E 038° 38' 015").
Sample extraction
The fresh leaves were distilled through hydro distillation by using Clevenger type apparatus for three hours and collected the pale yellow essential oil after drying with Anhydrous Sodium Sulfate. The essential oils were subjected to determine the physical property, chemical composition profile and antioxidant activity.
Physical properties of essential oils
The yields of the essential oils were calculated based on the formula described below.
Oil yield (v/w (%))=100 × Amount of distilled oil (ml)/Amount of fresh distilled leaf (g)
The specific gravity of the essential oil was measured by using Pycnometer. The refractive indexes of the essential oils were measured by Refractometer (Reichert, AR 200) and the Optical rotations of the essential oils were measured by a Polarimeter (Biobase, Authomatic).
Gas Chromatography-Mass Spectrometry (GC-MS) analysis
The GC-MS (Agilent model 7820 A) was used to determine the chemical composition profile of the essential oil. The instrument was conditioned with a split/ split less injector, MS detector (5977), and HP-5 SM capillary column (0.25 mm i.d. × 30 m × 0.25 μm film thickness). Injector was operated in split less mode with an injection volumes were 1 μL. Injector and detector temperatures were set at 250°C. Helium was used as carrier gas and controlled in constant flow mode at a linear velocity of 36 cm/sec. The oven was programmed to start at 50°C, which is held for 1 minute; then the temperature was ramped at 10°C/min to 200°C; then the temperature was ramped at 8°C/ min to 250°C, which was held for 3 minutes. Total running times for full analysis was 29.25 minutes.
Free radicals scavenging activity on 2, 2-diphenyl-1- picrylhydrazyl (DPPH)
The DPPH free radical scavenging activities of the essential oils were determined by the method described by Brand-Williams et al. [8]. Five concentrations (100, 50, 25, 12.5 and 6.25 μL/mL) of 50 μL samples were mixed with 5 mL of 0.004% (0.1 mM) methanol solution of DPPH. The mixture was incubated for 30 minutes at 37°C. After incubation, the absorbance of the mixture was read at 515 nm using UV-Vis spectrophotometer. Ascorbic acid was used as a positive control. Tests were carried out in triplicate and average values were taken. Inhibition of DPPH free radical was calculated by using the equation:
I (%)=100 × (Ao-As)/Ao,
Where Ao is the absorbance of DPPH solution (containing all reagents except the test sample), and As is the absorbance of the tested sample. The IC50 value which represented the concentration of the samples that caused 50% inhibition was determined based on the linear equation of concentrations of the Eucalyptus oils versus inhibition for all tested samples.
Hydro distillations of fresh leaves of Eucalyptus acquired an essential oil yield (v/w) of 1.75% (E. globulus) and 2.00% (E. citrodora ). This result is in agreement with the work reported by Bachheti [9], E. globulus located at eastern part of Ethiopia yielded 1.21% and Silva et al. [10], from Brazil origin obtained an average yield of 1.07% and 1.7% for E. globulus and E. citriodora, respectively. The physical properties of the essential oils were recorded as Specific gravity (0.92 g/mL and 0.91 g/mL), Refractive index (1.45952 and 1.45215) and Optical rotation (+8.155° and +0.559°) for E. globulus and E. citriodora, respectively (Table 1). These values were in accordance with the standard range of AFNOR [11] recommendations, which were Specific gravity (0.906-0.923), Refractive index (20°C) (1.4590-1.4670) and Optical rotation (0-8°) for E. globulus essential oil. A comparable results also reported by Loumouamou et al. [12] for physical property of E. citriodora essential oil from Congo Brazzaville origin which have Refractive index (1.4506-1.4525) and optical rotation (+2.38°-+6.25°) [13].
| Parameters | E. globules | E. citrodora | |
|---|---|---|---|
| Essential oil yield (% v/w) fresh based | 1.75 ± 0.21 | 2.00 ± 0.23 | |
| Specific gravity (g/mL) | 0.92 | 0.91 | |
| Refractive index (at 20°C) | 1.45952 | 1.45215 | |
| Optical rotation (O) | 8.155 | 0.559 | |
| FRSA | IC50 (µL/mL) | 864.59 | 186.42 |
| IC50 (mg/mL) | 795.42 | 169.64 | |
Table 1: Physical properties and FRSA activity of Eucalyptus essential oils. FRSA: free radical scavenging activity.
Essential oils of Eucalyptus were exhibited free radical scavenging activity against DPPH in dose-dependent manner with a ranging of 7.1-12.35% (E. globulus) and 9.8-30.45% (E. citriodora) for the tested concentrations of 6.25-100 μL/mL. Based on the linear equation of essential oil concentrations of Eucalyptus versus inhibition for all tested samples, IC50 values were determined as 795.42 mg/mL for E. globulus and 169.64 mg/mL for E. citriodora. The control Ascorbic acid had an IC50 value of 0.577 mg/mL (Figures 1 and 2). Eucalyptus citriodora essential oil had more potent to antioxidant activity against DPPH free radical. This is may be due to presence of Citronellol in the oil which is known to have a very good antioxidant potential [3].
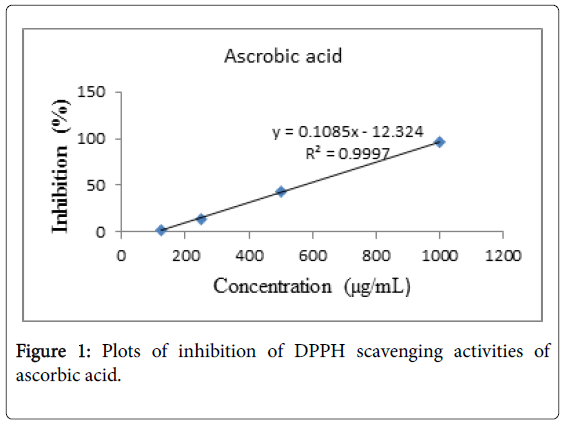
Figure 1: Plots of inhibition of DPPH scavenging activities of ascorbic acid.
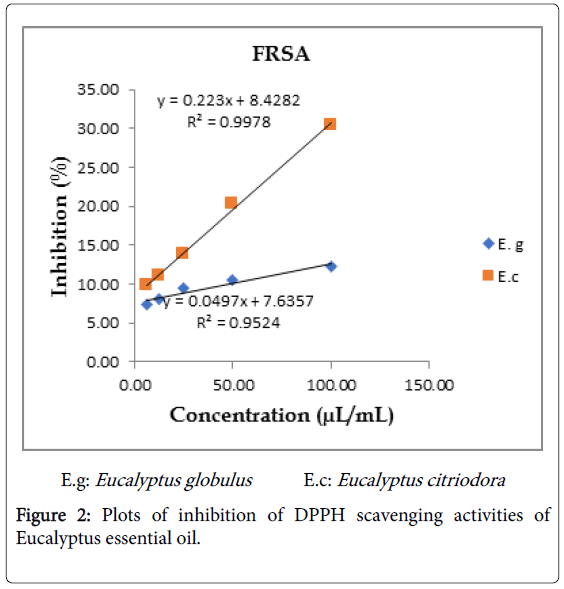
Figure 2: Plots of inhibition of DPPH scavenging activities of Eucalyptus essential oil.
The GC-MS analysis report of E. globulus essential oil revealed that five compounds were identified, of which 1, 8-Cineole (77.24%) and α- Pinene (10.54%) were the majorly found (Figure 3 and Table 2). Eucalyptus globulus essential oil from the like origin with different locations, 1, 8-Cineole (62.38%) and (57.5%) were reported by Bachheti [9] and Dagne et al. [3], respectively. A comparable 1, 8- Cineole content (85.5% and 85.8%) were reported by Silva et al. [10] and Boukhatem et al. [14] from Brazil and Algeria origin E. globulus essential oil, respectively. Irrespective of origin, 1, 8-Cineole content of E. globulus essential oil can vary between 54-95% [4].
Figure 3: GC-MS chromatogram of E. globulus essential oil.
| Peak No. | RT (min) | Name of compound | Molecular formula | Molecular weight (g/mol) | Composition (%) |
|---|---|---|---|---|---|
| 1 | 5.352 | 1R-α-Pinene | C10H16 | 136 | 10.54 |
| 2 | 6.918 | D-Limonene | C10H16 | 136 | 3.08 |
| 3 | 6.984 | 1,8-Cineole | C10H18O | 154 | 77.24 |
| 4 | 9.512 | α -Terpineol | C10H18O | 154 | 1.29 |
| 5 | 11.765 | Terpinolene | C10H16 | 136 | 2.23 |
Table 2: Chemical compositions of E. globulus essential oil.
A total of six compounds were identified from essential oil of E. citriodora from which Citronellal (73.86%) and Citronellol (14.13%) were dominantly comprised (Figure 4 and Table 3). In agreement results were reported by Dagne et al. [3] Citronellal (73.3%); Silva et al. [10] Citronellal (76.1%); Loumouamou et al. [12] with constituents of Citronellal (57.1-75.4%) and Citronellol (8-11%).
Figure 4: GC-MS chromatogram of E. citriodora essential oil.
| Peak No. | RT (min) | Name of compound | Molecular formula | Molecular weight (g/mol) | Composition (%) |
|---|---|---|---|---|---|
| 1 | 8.437 | trans-Chrysanthemal | C10H16O | 152 | 0.99 |
| 2 | 8.894 | Citronellal | C10H18O | 154 | 73.86 |
| 3 | 9.022 | Isopulegol | C10H18O | 154 | 3.21 |
| 4 | 10.001 | Citronellol | C10H20O | 156 | 14.13 |
| 5 | 11.758 | 2,6-octadiene,2,6-dimethyl | C10H18 | 138 | 6.42 |
| 6 | 12.85 | Caryophyllene | C15H24 | 204 | 1.39 |
Table 3: Chemical compositions of E. citriodora essential oil.
Eucalyptus is expressed as huge potentials for Ethiopian climatic conditions. Among, E. globules and E. citriodora are an exemplary to be established. The overall quality of the essential oils also comply the international specifications. Therefore, essential oil products which are obtained from potential area of Ethiopia can be used as medicine and perfumery industry.
I am gratefully acknowledged Mr. Abdela Befa for his collaboration during sample collection.
Citation: Abdo BM (2019) Physico-Chemical Profile and Antioxidant Activities of Eucalyptus globulus Labill and Eucalyptus citriodora Essential Oils in Ethiopia. Med Aromat Plants (Los Angeles) 8: 332. doi: 10.35248/2167-0412.19.8.332
Received: 12-Mar-2019 Accepted: 19-Mar-2019 Published: 28-Mar-2019 , DOI: 10.35248/2167-0412.19.8.332
Copyright: © 2019 Abdo BM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.