
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Review Article - (2021)Volume 12, Issue 2
Systemic Lupus Erythematosus (SLE) evolves into progressive and chronic inflammation of multiple joints and organs. No specific treatment exists for SLE which presents a diverse clinical polymorphism with unclear pathogenicity. Women at their pre-menopausal age are the most affected and early studies have reported the implication of estrogen in T cell abnormalities. Alteration of cAMP signaling in immune cells and target organs is emerging as cellular mechanism governing SLE disease progression. We recently reported that activity and expressions of PDE4, the major cAMP hydrolyzing enzyme were deregulated in kidney of lupus prone mice. Therefore, PDE4 inhibitors may exert anti-inflammatory effects on several immunocompetent cells including T and B lymphocytes, and macrophages. Several PDE4 inhibitors achieved good therapeutic values as potent anti-inflammatory compounds for the treatment of chronic inflammatory diseases including Crohn's disease, autoimmune disease (lupus), COPD, and neurodegenerative diseases. This review will discuss the mechanism of NCS 613, a new cAMP elevating agent in preventing systemic chronic inflammation in SLE. This PDE4 inhibitor is believed to reduce abnormal systemic inflammation orchestrated by overreactive T cells that stimulate autoantibodies production by autoreactive B cells and proinflammatory mediators release by macrophages. Ultimately, NCS 613 improve survival and overcome nephritis in mice and prevent inflammatory cytokines release in human stimulated leucocytes. PDE4 inhibition is a promising therapeutic target to tackle chronic inflammatory disease of different pathogenicity.
cAMP signaling; PDE4 inhibitors; Chronic inflammation; Lupus nephritis
AC: Adenylyl-Cyclase; AKAP: A-Kinase Anchoring Protein; AMP: 5' Adenosine Monophosphate; CREB: cAMP Response Element Binding Protein; cAMP: 3',5'-Cyclic Adenosine Monophosphate; cGMP: 3',5'-Cyclic Guanosine Monophosphate; COPD: Chronic Obstructive Pulmonary Disease; COX: cyclooxygenase; PDE: Cyclic Nucleotide Phosphodiesterase; PBMCs: Human Peripheral Blood Mononuclear Cells; ERK: Extracellular Signal-Regulated Kinase; HARBS: High Affinity Rolipram Binding Site; IP3: Inositol Triphosphate; IL: Interleukin; MIP: Macrophage Inflammatory Protein; LPS: Lipopolysaccharide; NF-κB: Nuclear Factor Kappa B; NFAT: Nuclear Factor of Activated T Cells; TNF-α: Tumor Necrosis Factor Alpha; SLE: Systemic Lupus ErythematosusKeywords: Vaccine; Alum; MPL; Adjuvant; Staphylococcus aureus; Sepsis
Systemic Lupus Erythematosus (SLE) is a polymorphic and heterogenic autoimmune disease that leads to progressive and chronic inflammation of multiple joints and organs. Generated autoantibodies target several nuclear compartments including spliceosomes, DNA, and phospholipids [1]. Women at their pre- menopausal age are the most affected. SLE is a complex disease with broad spectrum of clinical manifestations; however its pathogenicity remains fairly understood [2]. Early studies have incriminated the role of estrogen in SLE pathogenicity based on the high prevalence in women, particularly those of African descents. It has been reported that estrogen act on their receptors to increase the activation of T cells from women with SLE. This amplifies the interactions between T and B lymphocytes, then the activation of B cells to produce autoantibodies [3]. T cells also stimulate macrophages to release variety of inflammatory mediators. Glomerulonephritis, polyarthritis, neuropsychiatric disorders, and dermatitis are the most frequent clinical complications of SLE. Most patients develop glomerulonephritis and renal complications due to the deposition of immune complexes in the nephron [4].
Macrophagic cell infiltration and the nuclear targets of cytokines and antibodies are the causes of kidney damage. There is no specific treatment for SLE, which is addressed with symptomatic prophylaxis, such as corticoids and immunosuppressive drugs [5]. For a long time, steroidal anti-inflammatory drugs (glucocorticoids) and non-steroidal anti-inflammatory drugs (COX inhibitors) have contributed to the management of chronic inflammatory diseases including SLE. However, their therapeutic benefits are tarnished by the occurrence of potentially serious gastric and hemorrhagic side effects [6]. Moreover, these first line therapies lack efficacy in the case of chronic inflammation.
From a cellular and molecular point of view, SLE is characterized by alterations in certain signaling pathways leading to deregulation of the immune system. For instance, intracellular calcium handling is unbalanced in T lymphocytes which lead to overactivity of T cells. In addition, a low intracellular cyclic adenosine 3′,5′-monophosphate (cAMP) level is reported to be associated with impaired cAMP- dependent phosphorylation of endogenous substrates in T lymphocytes from patients with active SLE [7,8]. Cyclic nucleotide phosphodiesterases (PDEs) that specifically hydrolyze cAMP and cGMP play an important role in the normal and pathological control of the cellular response. It has been shown that a high levels of intracellular cAMP in inflammatory or immunocompetent cells leads to a reduction of inflammatory mediators including chemokines, cytokines, and reactive oxygen species [9-11]. Alterations of PDE4 expressions in kidney of lupus prone mice and peripheral blood mononuclear cells (PBMCs) of patients were previously reported by our group [12,13]. PDE4 variants are associated with various A-kinase anchoring proteins (AKAPs), allowing PDE4 to orchestrate signaling crosstalk, desensitization, and cAMP compartmentalization [14,15]. This family of enzymes which participates in more than 70% of total cAMP hydrolysis in PBMCs, is involved in the control of inflammatory responses [12,16]. In other word increase in PDE4 hydrolytic activity and low intracellular cAMP levels strongly correlate with inflammation [17]. PDE4 inhibitors exert immunosuppressive effects on inflammatory cells by blocking the secretion of cytokines (TNFα, IL-6, IL-1…), the migration of leucocytes to inflammatory sites [11,12]. This review discusses our latest understanding of cAMP signaling in chronic inflammation and the pharmacological inhibition of PDE4 in the management of chronic inflammation and autoimmune diseases.
cAMP was discovered in the 1950s as a second messenger in the hormonal regulation of epinephrine metabolism in the heart and liver of mammals [18]. cAMP is ubiquitously synthesized from adenosine triphosphate (ATP) by adenylyl cyclases. Adenylyl cyclases are activated by various signaling molecules through stimulatory G protein-coupled receptors (Gs) and inhibited by inhibitory G protein-coupled receptors (Gi). Downstream of surface membrane receptor activation, 11 families of PDEs regulate cAMP and cGMP signaling by breaking down the local cyclic nucleotide concentrations to basal levels [19,20]. Among cAMP- PDE, PDE4 is the largest of the family. It is comprised of 4 genes (PDE4A, PDE4B, PDE4C, and PDE4D) with various alternative mRNA splices encoding long and short PDE4 isozymes, resulting in 35 different proteins [21]. In fact, the hydrolytic activity of PDEs against cAMP is ten times greater than that of synthesis, and therefore the levels of cAMP in the cells are determined by PDEs activities.
In mammals, cAMP mainly targets cAMP-dependent protein kinase A (PKA), exchange proteins activated by cAMP (EPACs), and cyclic nucleotide gated ion channels (CNGs and HCNs). More recently, a fourth potential target of cAMP has been identified, which is PDE10 [22]. PKA can directly phosphorylate several target proteins and thus increase or decrease their activities. The regulatory domains of PKA are important for its subcellular localization through anchoring proteins (AKAPs). AKAPs bind to regulatory subunits of PKA and organelle components in the membrane, cytoskeletal and nuclear compartment. AKAPs promote the anchoring and the formation of complexes called signalosomes (i.e inflammasomes) bringing together adenylyl-cylases, PKA, phosphodiesterases and other effectors such as Epac (Figure 1). One of the major targets of PKA is cAMP responsive element binding (CREB) which controls the transcription of proinflammatory genes. Therefore, cAMP is an important modulator of various cellular pathways including inflammatory response.
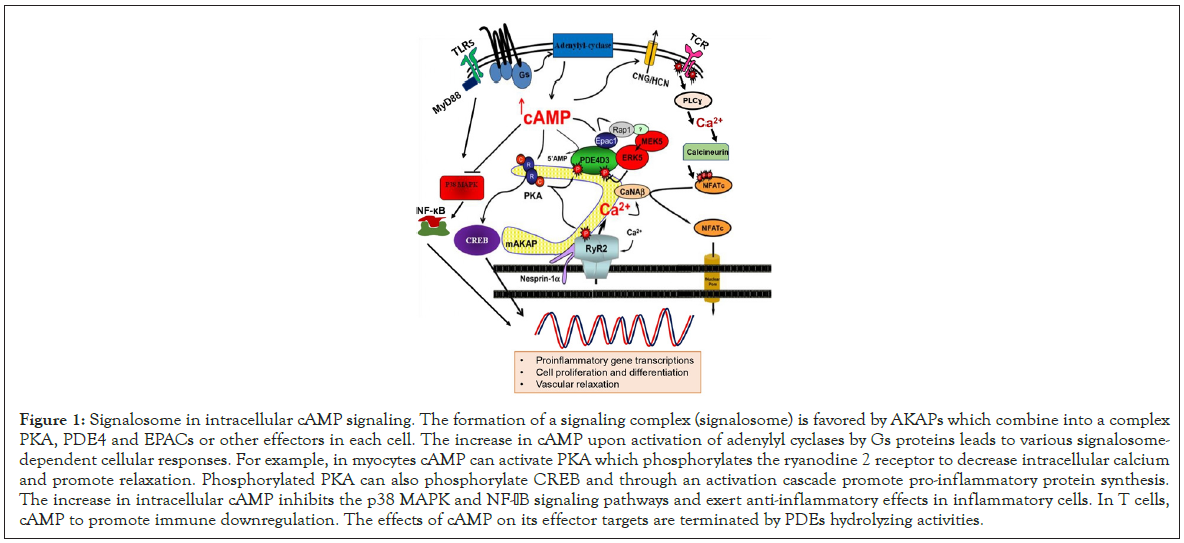
Figure 1: Signalosome in intracellular cAMP signaling. The formation of a signaling complex (signalosome) is favored by AKAPs which combine into a complex PKA, PDE4 and EPACs or other effectors in each cell. The increase in cAMP upon activation of adenylyl cyclases by Gs proteins leads to various signalosome- dependent cellular responses. For example, in myocytes cAMP can activate PKA which phosphorylates the ryanodine 2 receptor to decrease intracellular calcium and promote relaxation. Phosphorylated PKA can also phosphorylate CREB and through an activation cascade promote pro-inflammatory protein synthesis. The increase in intracellular cAMP inhibits the p38 MAPK and NF-aB signaling pathways and exert anti-inflammatory effects in inflammatory cells. In T cells, cAMP to promote immune downregulation. The effects of cAMP on its effector targets are terminated by PDEs hydrolyzing activities.
PDE4 are predominantly found in the brain, inflammatory and immune cells, cardiovascular tissues, and smooth muscles [23,24]. They are insensitive to cGMP and specifically inhibited by Rolipram which was designed as an antidepressant. PDE4 specifically hydrolyze cAMP with a Km=2-4 μM, they are distinguished in their structures by a region called upstream conserved region (UCR) located in the N-terminal part. Long isoforms have UCR1 and UCR2 whereas short isoforms have only UCR2 [16]. Super-short isoforms have only part of UCR2 (truncated form). The short- term regulation of PDE4 takes place by phosphorylation of PKA at UCR1 which increases the catalytic activity of PDE4. In the case of prolonged increase in cAMP levels in response to a stimulus, PKA-dependent phosphorylation allows a feedback mechanism [25]. Phosphorylation by PKA can lead to the dimerization of long isoforms of PDE4 and to their activations. Phosphorylation of PDE4 by ERK2 in the C terminal region activates short forms (PDE4D) and inhibits long forms. Prolonged accumulation of cAMP may induce long-term regulation of PDE4 relative to induction of new proteins by de novo synthesis dependent on phosphorylation of CREB by PKA [16]. Sumoylation is a post- translational modification resulting in the covalent binding of one or more SUMO proteins (for Small Ubiquitin-like MOdifier) to a lysine acceptor of a target protein. It was shown that the sumoylation of long isoforms of PDE4 (4A and 4B) increases their activation by phosphorylation of PKA and promotes the repression of their inhibitions by phosphorylation by ERK [26].
Chronic inflammatory diseases remain a public health concerns including inflammatory bowel (Crohn's disease), autoimmune (lupus), recurrent pulmonary (asthma and COPD), and neurodegenerative diseases (Alzheimer's, multiple sclerosis, schizophrenia). The most PDE4 isoforms expressed in leukocytes are PDE4B2 (short isoform) and PDE4D3 and D5 (long isoforms). Long PDE4D isoforms predominate in monocytes, whereas short PDE4B isoforms predominate in macrophages [26]. In mice deficient in PDE4B, stimulation of immune cells by lipopolysaccharide (LPS) revealed that this isoform plays an essential role in TNF-α production by peripheral leukocytes [27].
cAMP is the most second messenger extensively studied in T lymphocyte proliferation, differentiation and activation. It has been reported that recruitment of PDE4 to lipid rafts can overcome cAMP-mediated inhibition of T cell activation which is important in sustaining T cell immune responses [28]. PKA in T cells targets transcription factors such as cAMP response CREB, nuclear factor of activated T cells (NFAT), and nuclear factor κB (NF-κB) to reducing the production of pro-inflammatory cytokines (IFN-ɣ, TNF-α, and IL-1β), T cell proliferation and T cell activation [12,28,29]. cAMP elevating agents further drive the development of T regs to maintain immunological homeostasis by suppressing the innate immune responses [30]. It is accepted that elevated cAMP levels attenuate the T lymphocyte mediated immune response; thereby PDE4 inhibitors are attracting immunomodulators. Phosphodiesterase type 4 in neutrophils involved in the production of IL-8, leukotriene B4, and superoxide anions facilitating degranulation and chemotaxis of neutrophils [31]. PDE4 induce the expression of the neutrophil β2-integrin (Mac-1) allowing adhesion of neutrophils to vascular wall endothelium [32]. cAMP plays a key role in the regulation of activated macrophage inflammatory responses. In dendritic cells, cAMP has been shown to suppress the release of pro-inflammatory mediators (TNF-α, IL-17, IFN-γ) while promoting anti-inflammatory IL-10 release. Functionally, elevated cAMP levels in dendritic cells induce Th2 immunity [33]. Elevation of intracellular cAMP enhances IgE production by B cells and favor Th2 immune response. The role of cAMP signaling in regulation of innate and adaptive immunity was described in detail in previous review papers [34,35]. Pharmacological manipulation of cAMP levels through either PDE4 inhibition or cAMP-agonist administration have been widely shown to dramatically reduce inflammatory response and phagocytic function of macrophages and other innate immune cells [36].
Suppressing the functions of inflammatory cells remains an interesting therapeutic strategy to globally control inflammation in systemic autoimmune diseases. Elevated intracellular cAMP in inflammatory cells bears the therapeutic benefits of suppressing the expression of pro-inflammatory cytokines (TNF-α and IL-1), chemokines (MIP-1α and MIP-1β), and the pro-inflammatory lipid mediator (leukotriene B4) [29]. Interestingly, cAMP enhances the production of the anti-inflammatory cytokine IL-10 in PBMCs. The development of PDE4 inhibitors are opening new avenues for the management of chronic inflammatory diseases. Theophylline, which is a non-selective PDE inhibitor has been on the market for a very long time (before the discovery of PDEs) for the treatment of asthma and rolipram for the treatment of cognitive impairment. Although PDE4 inhibitors have shown strong anti-inflammatory effects, their therapeutic benefits are hampered by side emetic effects related to their binding to the “high-affinity rolipram binding site” (HARBS) [36]. Interestingly promising PDE4 inhibitors are emerging for the treatment of variety of chronic inflammatory diseases and their emetic effects are addressed alongside with drug development.
The structure-activity approach allowed the synthesis of several new generation PDE4 inhibitors with fewer emetic side effects. Roflumilast is used in the clinic as an add-on therapy to bronchodilator treatment for the severe chronic obstructive pulmonary disease [37]. Cilomilast was also developed for the treatment of chronic obstructive pulmonary disease and asthma. Other PDE4 inhibitors are currently being developed for autoimmune diseases such as rheumatoid arthritis, allergic skin diseases, and psoriasis. Among them, Apremilast was recently approved for the treatment for psoriatic arthritis [38]. It may also be useful for other immune system related inflammatory diseases. Ibudilast was developed as neuroprotective and bronchodilator drug and used mainly in the treatment of asthma and stroke [39]. While Ibudilast inhibits PDE4 to the greatest extent, significant inhibitory effects have been found with other PDE subtypes depending on the dose. Ibudilast has also demonstrated the potential therapeutic value for oral treatment of other forms of multiple sclerosis (MS) and other neurodegenerative disorders [40]. Piclamilast is a second generation selective PDE4 inhibitor which exhibits structural functionalities of cilomilast and roflumilast [41]. Piclamilast is designed for the treatment of conditions such as chronic obstructive pulmonary disease, bronchopulmonary dysplasia, and asthma. Most of these therapeutic agents rely on decreasing the activities of PDE4 to reduce the inflammatory responses in multiple cell types from many disease conditions [42].
Other new PDE4 inhibitors are under preclinical development to tackle severe chronic inflammation. For example, Arofylline (LAS31025), a selective PDE4 inhibitor with anti-inflammatory activity was evaluated in atopic dermatitis [43]. Our group has intensively investigated the effects of NCS 613, a selective PDE4 inhibitor on chronic inflammatory conditions [11,17,44]. Finally, PDE4 inhibitors are gaining more attention in field immuno- inflammation and neurologic disorders [45]. For the ability to increase the memory capacity, these inhibitors are becoming attractive therapeutics for Alzheimer's disease.
Currently, SLE is managed by symptomatic treatment, such as corticoids, as well as antibodies and peptides targeting T and B cells, anti- TNFα and anti-interleukin-10 antibodies [46]. No specific treatment for SLE including low molecular weight drug exists. Emerging research from our lab aimed at investigating the evolution of PDE4 activity and expressions in MRL/lpr lupus- prone mice which develop lupus nephritis with similar symptoms like in human [11-13]. We reported that disease progression in mice is associated with significant changes in kidney PDE4 activity and reduced intracellular cAMP levels. Kidney PDE4 activity was the major cAMP hydrolyzing enzymes and PDE4B remained increase during disease course [11]. Consistent with earlier observations that cAMP-PDE inhibitors improve sodium excretion in rats with cirrhosis [47], NCS 613 beneficially impacted proteinuria, the hallmark of lupus and overcame disease progression in MRL/lpr mice. NCS 613 did not stimulate the gastric acid secretion suggesting that this compound may produce fewer gastrointestinal side effects. When PBMCs from MRL/lpr were stimulated, NCS 613 inhibited basal and LPS-induced TNF-α secretion. Studies carried on human PBMCs revealed the upregulation of PDE4B and PDE4C upon LPS stimulation which were reduced by NCS 613 treatment. In PBMCs from both healthy donors and SLE patients, NCS 613 targets PDE4 and indirectly p38 MAPK and NF-κB, leading to reduction of proinflammatory cytokine release [12,17]. Consequently, NCS 613 abolished LPS-induced inflammation in PBMCs by reducing IL-6, IL-8, and TNF-α cytokines. It is understandable that the novel PDE4 inhibitor might also reduce T and B lymphocytes activities. In 80% of SLE patients, CD3ζ molecule is replaced by a Fcγ receptor which is normally associated with the syk kinase. Absent in the abnormal T lymphocyte, syk increases the concentration of intracellular calcium by 100 times [48,49]. The increased activity of CD3ζ-hydrolyzing caspase-3 strongly contributes to impaired TCR/CD3 signal transduction [50]. Abnormal calcium handling leads to T cells overactivity. Overactive T lymphocytes in turn activate autoreactive B cells to produce autoantibodies and macrophages to secrete a high amount of TNF-α. In this regard, NCS 613 have a potential to overcome abnormal immune activation (Figure 2). Administration of NCS 613 significantly reduced systemic inflammation and autoantibodies deposition in the kidney [11]. This cAMP elevating agent was able to restore to the normal level the altered PDE4 expressions. Evidence was provided that increasing intracellular cAMP in immune cells has a broad impact on suppressing aberrant proinflammatory signaling. Interestingly, treatment with PDE4 inhibitor such as NCS 613 provides therapeutic strategies to block TNF-α and B cells as previously targeted with TNF-α receptor agonist and rituximab respectively. Worthwhile, NCS 613 may also promote T regulatory cell proliferation while blocking T cell overactivity.
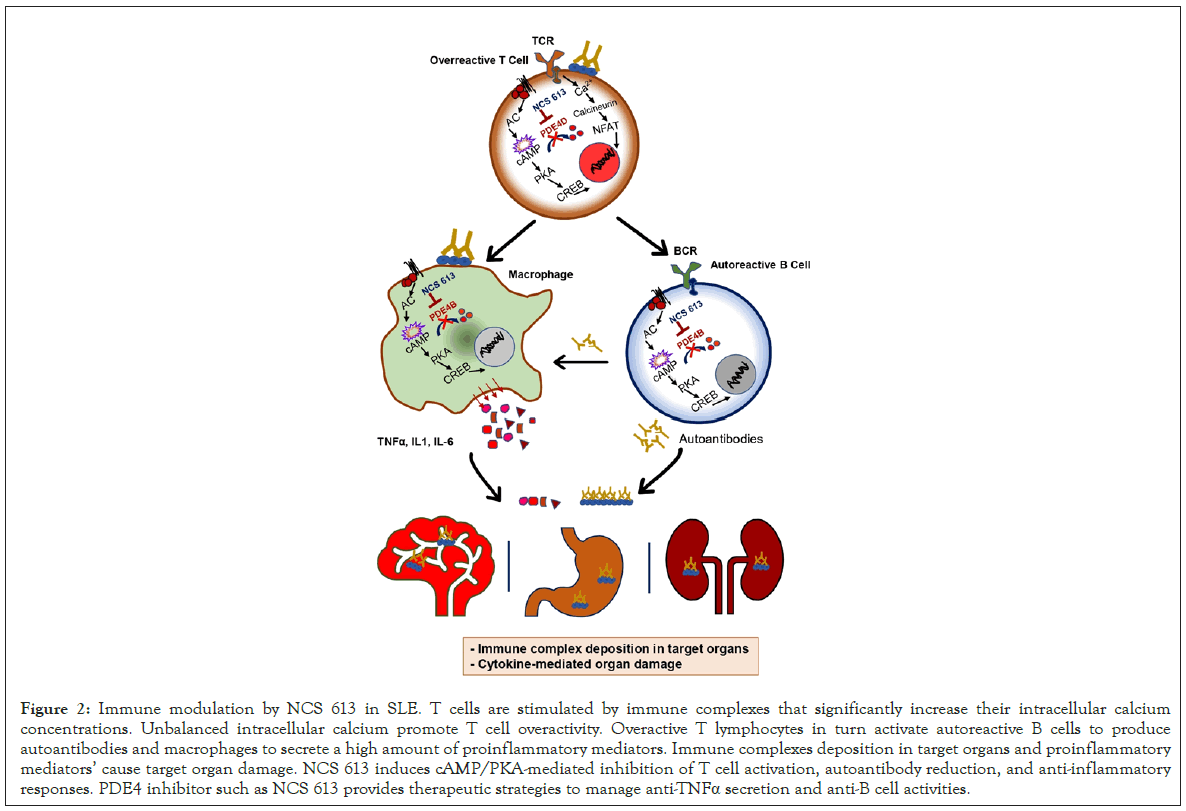
Figure 2: Immune modulation by NCS 613 in SLE. T cells are stimulated by immune complexes that significantly increase their intracellular calcium concentrations. Unbalanced intracellular calcium promote T cell overactivity. Overactive T lymphocytes in turn activate autoreactive B cells to produce autoantibodies and macrophages to secrete a high amount of proinflammatory mediators. Immune complexes deposition in target organs and proinflammatory mediators’ cause target organ damage. NCS 613 induces cAMP/PKA-mediated inhibition of T cell activation, autoantibody reduction, and anti-inflammatory responses. PDE4 inhibitor such as NCS 613 provides therapeutic strategies to manage anti-TNFa secretion and anti-B cell activities.
PDE4 pharmacology research is gaining more interest and selective PDE4 inhibitors are emerging as potent anti-inflammatory drugs. Abroad rang of disease are in scope of treatment with PDE4 inhibitors to mitigate chronic inflammation including Crohn's disease, autoimmune diseases (lupus), COPD, and neurodegenerative diseases. Regarding autoimmunity, PDE4 play a key role in immune cells involved in disease pathogenesis. Body of evidences suggests that immune complex deposition and macrophage infiltration into a target organ may be the root cause of kidney failure in SLE. The pharmacological inhibition of PDE4 activity can impact T cells overactivation and in turn reduce auto- reactive B cell activity and macrophage migration. Inhibition of systemic inflammation by NCS 613 in preclinical study has proven to improve proteinuria and overcome disease progression in lupus prone mice. Future research should focus on understanding the role PDE4 in T cell overactivation, autoantibody secretion by B cell, and macrophagic infiltration into kidney. Understanding the functional outcome of the pharmacological inhibition of PDE4 in autoimmunity may lead to the development of novel treatments for chronic inflammation. It is, therefore, imperative that the mechanisms that promote anti-inflammatory responses by cAMP elevating agents are delineated from those that promote emetic side effects.
The author declares no conflict of interest.
Citation: Yougbare I (2021) Pharmacological Inhibition of cAMP Signaling is an Attractive Therapeutic Strategy for Management of Chronic Inflammatory and Autoimmune Diseases. J Clin Cell Immunol. 12:613.
Received: 05-Feb-2021 Accepted: 19-Feb-2021 Published: 26-Feb-2021 , DOI: 10.35248/2155-9899.21.12.613
Copyright: © 2021 Yougbare I. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.