
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2022)Volume 12, Issue 5
Background: Early in the COVID-19 pandemic, no evidence proven therapeutics were available, and thus participation in a clinical trial was often the only way to access experimental therapeutic options. In the US, participation in medical research is low, and patient stated factors driving non-enrollment decisions are poorly characterized. Thus, the aim of this study was to identify patient and legally-authorized representative reported reasons for declining enrollment in a COVID-19 therapeutics trial identify potential strategies for addressing barriers in future investigations.
Methods: As part of a pragmatic randomized trial during the period from 4/10/20-2/3/21, SARS-CoV-2 positive inpatients with moderate to severe disease were screened for eligibility. If eligible patients declined to participate, they were asked an open-ended question about the reasons behind their decision. Qualitative responses were analyzed using a directed content analysis approach; responses were categorized using previously defined factors that have contributed to decisions not to enroll in other clinical therapeutics trials, primarily conducted in oncology. To evaluate the impact of external factors, such as publication of evidence proven therapeutics options, enrollment rates by time period were assessed using simple descriptive statistics: time period 1 (before June 25, 2020) before any evidence-based treatments were available; time period 2 (June 25 August 26), after dexamethasone was recommended but before tocilizumab was recommended against; and time period 3 (August 27 March 5, the study end date).
Results: N=417 patients were screened, and 162 patients met eligibility criteria. Of these, 53 (32.7%) consented to enroll. A total of 102 (62.9%) patients declined to participate, and 7 were unable to give consent and were excluded. Patient reported reasons for non-enrollment were limited perceived benefit, competing priorities, physician or family influence and presence of comorbidities leading to perception of increased risk of participation. Several patients reported that their decision was influenced by physician or family recommendation to decline, which was reported as physician lack of support for participation due to the presence of comorbidities or physician perceived lack of benefit given clinical improvement prior to study enrollment.
Enrollment varied substantially by study period. During the first time period (prior to dexamethasone), the enrollment rate was 10/11 (91%) and during the period from August 25 until the end of the trial, after some other studies suggested lack of effectiveness of IL-6 receptor inhibition for management of severe COVID-19, the enrollment rate fell to 43/144 (30%) (P-value, <0.0001).
Conclusions: Understanding reasons and attitudes driving decisions to decline enrollment may help investigators address them during the recruitment process and increase participation in clinical trials in the US.
COVID-19; Dexamethasone; IL-6 Receptor inhibitor; Therapeutics trial; Clinical trial; Enrollment rates; Clinical trial participation
Since the identification of COVID-19 in early 2020, over 500 million people have become infected, and an estimated 6.2 million deaths have occurred worldwide [1]. Given rapid spread and high case fatality rate early in the pandemic, there was an urgent need to identify effective therapeutics to improve clinical outcomes. In theory, in the setting of a global emergency with limited treatment options available, engagement in clinical trials might be higher than is typical.
Insufficiently powered trials and low patient enrollment remain a concern despite the recognized need to improve enrollment [2]. Pre-pandemic, and outside of a state of emergency, public participation in medical research was already low in the United States, estimated at 10% [3]. Low recruitment rates are the most common reason for terminating clinical trials in the United States [4,5]. The reasons for low enrollment are multifactorial and include limited perceived benefit, fear of the unknown or adverse effects, physician influence and presence of other comorbidities, inconvenience, and access to research information [6,7]. During the pandemic, patient enrollment also faced a new set of challenges including limited in-person contact with potential participants, lower number of on-site research staff, and a shift to electronic informed consent [8].
Given the need to identify strategies to improve participation in COVID-19 clinical trials and overall in the United States, we report qualitative patient responses who were eligible to participate in a Sarilumab COVID Therapeutics Trial across five Veterans Affairs (VA) Medical Centers in the Veterans Integrated Service Network (VISN)-1 clinical trials network but declined enrollment [9]. We aimed to identify common themes behind patient decision making with regard to enrollment and to determine if availability of different treatments changed the decision to participate, and to evaluate the impact of the time period (pre or post availability of other medications) on enrollment rates. Understanding factors that influence decisions about participating in COVID therapeutic trials may help increase participation in future studies.
As part of an open label, adaptive, pragmatic, embedded randomized clinical trial across five Veterans Affairs (VA) Medical centers in the Northeast from April 10,2020 and February 3, 2021, patients were screened for potential eligibility and participation (NCT04359901) [9]. The study intervention was a one-time dose of a medication (sarilumab, delivered subcutaneously). Outcomes were assessed using electronic health record data only; no additional participation was required of patients after the one time intervention.
Patients with a positive SARS-CoV-2 diagnostic test (PCR or antigen testing) within 4 weeks of hospitalization for moderate to severe COVID-19 and with symptom duration of <14 days were potentially eligible. Given the adaptive nature of the trial, enrollment criteria changed over time and patients with more moderate disease became eligible later during the trial. The trial initially opened at one site and then expanded to include a total of five VA sites in the VISN-1 clinical trials network.
Consent processes varied by trial site and ranged from fully inperson to fully remote processes; extensive details of the fully remote process are previously published [10]. In all cases, potentially eligible patients received an informed consent packet and were then contacted by a member of the study team who discussed the study in more detail. Patients or their legal representatives who indicated that they were not interested in enrolling were then asked an open ended question about their reason(s) for opting not to enroll. If the patient did not offer a response to an open ended question, the patient was offered several categories of options and asked if their reason fell into any of the broad categories. Early in the trial, the data collection process for reasons for non-enrollment was not formalized, and thus more complete data are available for later time periods.
Enrollment rates by time period were assessed using simple descriptive statistics and compared via Chi-squared test, divided into the time before any evidence based treatments were available: Time period 1 (before June 25, 2020), before any evidence based treatments were available; time period 2 (June 25 August 26), after dexamethasone was recommended but before tocilizumab was recommended against; and time period 3 (August 27 March 5, the study end date), the period after IL-6 receptor inhibition was recommended against [11]. Given the very low number of eligible patients during Time period 2, only time periods 1 and 3 were compared [12].
Qualitative responses for opting not to enroll were analyzed using directed content analysis and categorized based on previously defined key concepts demonstrated in other contexts to contribute to non-enrollment [13,14]. In addition, themes that emerged through patient responses were categorized and analyzed. After the data were coded, two analysts (WBE, SRD) met to review categorization, and differences were adjudicated. Responses from patients who were screened but not eligible for participation were not included in the analysis.
The study was approved by the VA Boston IRB (#3305) prior to data collection and analysis.
N=417 patients were screened, and 162 patients met eligibility criteria. Of these, 53 (32.7%) consented to enroll. A total of 102 (62.9%) patients or their legally authorized representatives declined to participate, and 7 were unable to give consent and were excluded. Enrollment varied across study sites; two sites accounted for the majority of screened and enrolled patients (Table 1). Over the five study sites, enrollment varied from 5 to 100 percent. Enrollment also varied significantly by study period (Figure 1). Of the 155 patients either declining or consenting to enrollment, 11 were approached in time period 1 and 144 were approached in time period [2].
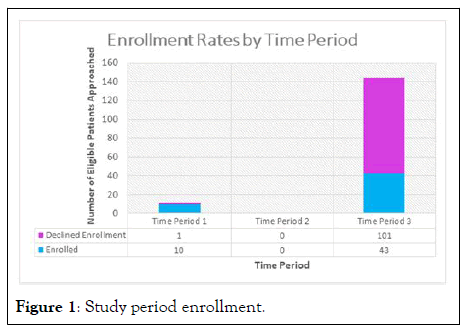
Figure 1: Study period enrollment.
| Variable | Eligible (N=155) | Enrolled (%) (N=53) | Declined to Enroll (%) (N=102) | p-value* |
|---|---|---|---|---|
| Site | ||||
| Site 1 | 10 | 4 (40%) | 6 (60%) | 0.22 |
| Site 2 | 8 | 8 (100%) | 0 | |
| Site 3 | 75 | 26 (35%) | 49 (65%) | |
| Site 4 | 42 | 14 (33%) | 28 (67%) | |
| Site 5 | 20 | 1 (5%) | 19 (95%) | |
| Study period | ||||
| Pre-dexamethasone guideline-recommendation | 11 | 10 (91%) | 1 (9%) | <0.0001 |
| Post-dexamethasone guideline-recommendation | 144 | 43 (30%) | 101 (70%) | |
*Pearson Chi-squared
Table 1: Comparison of patients who opted to enroll versus declined to enroll.
During the period before the first effective treatment (dexamethasone) was identified in clinical trials, the enrollment rate was 10/11 (91%) and during the period from August 25 until the end of the trial, after initial reports suggested lack of effectiveness of IL-6 receptor inhibition for management of severe COVID-19 and the treatment received a “recommendation against, except in the setting of a clinical trial,” the enrollment rate fell to 43/144 (30%) (Chi-squared p-value, <0.0001).
A summary of qualitative responses is presented in Table 2. Patient reported reasons for non-participation fell into two broad categories: Patient factors and external factors, such as physician influence. Under these two major categories, several sub-themes were identified, including patient concerns about the study drug and participation in clinical research in general, concerns about how underlying health and comorbidities might impact safety of participation, competing priorities and external factors, and external advice and influence from family members and clinicians.
| Themes | Examples/Patient statements |
|---|---|
| Perceived limited benefit/Distrust | Doing well on current treatment |
| Didn’t want to be a guinea pig | |
| Internet research and thinks it’s just another thing we are trying that doesn’t work based on what he read | |
| Patient does not believe in COVID and that it causes pneumonia and does not think it was cause for admission | |
| Don’t want to risk getting worse | |
| Risk of getting more medication | |
| Risks of study drug | |
| It is experimental and don’t know if it works | |
| Been in too many research trials | |
| Competing priorities/External influences and stressors | Worried about wife, who was admitted to hospital with COVID |
| Brother died of COVID 2 weeks prior and too overwhelmed to participate | |
| A lot going on and very stressed and doesn’t want another variable to that stress | |
| Too much going on right now | |
| Already enrolled in two other trials | |
| Clinician/Relative recommendation | Patient/MD satisfied with current treatment plan |
| ID physician recommended not to participate | |
| Family member against patient’s participation | |
| Doctor told him that he was doing better and he did not need to participate | |
| Daughter felt trial would be too much | |
| Comorbidities/Concern about impact of study drug by treating physicians/MD satisfied with current treatment plan | Schizophrenia with history of psychiatric decompensating |
| Recent aspiration pneumonia | |
| History of coccidioidomycosis | |
| History of psychosis* | |
| Concern about kidney issues | |
| Advanced age | |
| Admitted for stroke** | |
| Per infectious diseases, patient has sepsis/open wound | |
| On medications for previous kidney transplant, physician determined not a good idea |
*Concern that the patient would not be able to consent given underlying psychiatric diagnoses.
**Patient had mild COVID-19 and was primarily admitted for management of stroke and felt not to be eligible for the study.
Table 2: Key themes and examples reported by patients who opted to decline clinical trial participation.
Many patients or their legal representatives reported a perception that the treatment being tested in the trial offered limited benefit and distrust about participation in clinical research. This was expressed as concerns about not wanting to “be a guinea pig” and perceived potential harms of the medication without proven benefit. Presence of comorbidities that were actively complicating their clinical course or posing higher risk for decompensating should they receive sarilumab also impacted patient decision making. Competing priorities included outside stressors that were unrelated to the current disease status of the patient, such as illness in family members that made consideration of participation in a clinical trial overwhelming.
In addition to patient driven factors, patients reported that physician or family recommendation to decline was a major driver of their decision making and attitude regarding clinical trial participation. Patients reported being influenced by their clinicians, who relayed concerns to patients about the presence of comorbidities or and perceived lack of benefit of treatment for various reasons, including clinical improvement rendering additional treatment unnecessary.
As part of a multi-center, embedded, randomized controlled trial, we assessed and identified patient reported key reasons behind non-participation in a COVID-19 therapeutics trial [9]. The most common patient or legally authorized representativereported reasons for opting not to participate in this clinical trial included limited perceived benefit, competing priorities, physician or family influence and presence of comorbidities leading to perception of increased risk of participation. Based on enrollment rates before and after publication of the clinical trial demonstrating the effectiveness of dexamethasone and after release of early studies that suggested a lack of benefit to IL6-R therapies, the availability of other medications and the national treatment guidelines also impacted decision making, despite allowance for use of the medication in the setting of a clinical trial in the official recommendations.
Identifying patient and/or legally authorized representative reasoning behind clinical trial non-participation and mapping these factors to strategies that may address these patient concerns may help to improve participation in clinical trials. In addition, data may be used to develop informed consent strategies and clinical trial designs that facilitate participation. Notably, this trial only required a one-time intervention delivery without post-discharge follow up, and thus the burden on patients for ongoing trial activities was extremely low; this low burden may have contributed to the relatively high enrollment rate compared to other studies in the United States.
Data on patient reported reasons in the US for not participating in clinical trials is limited, and most of the existing data is focused on clinical trials in oncology. These prior studies suggest that physician attitude and enthusiasm about the trial, and about the therapeutic agent in question, is one of the most important drivers of patient decision making, either by not offering the trial to patients or expressing concerns about comorbidities that impact the safety of participation [15-17]. These themes were also found in our study. Limited perceived benefit or distrust has previously been shown to limit enrollment in clinical trials, particularly in underrepresented groups, which may have been amplified given the rapidly changing landscape of available COVID-19 therapeutic agents.
To our knowledge, patient reasons for opting not to participate in COVID-19 therapeutics trials in the United States have not been previously reported or explored, however, fewer patients have enrolled in US clinical trials than in clinical trials in other settings and countries, most notably the UK [18]. Although not specifically addressed in this qualitative study of patient reported factors, our experience running the clinical trial suggests that the long and cumbersome, often remote, informed consent process may have served as a deterrent, both for patients and their providers. In total, the remote consent process was estimated to take 6-8 hours and, due to infection prevention measures, required multiple complicated document transfers to obtain consent and enroll, in a clinical trial involving a single therapeutic agent. This process is in stark contrast to the process in the UK recovery trial, in which a simplified consenting process was permitted, and one enrollment process was used for multiple agents. Recently published pragmatic designs with limited patient and provider burden for participation, such as the adaptable trial, also suggest that simplifying processes for both patients and providers may also improve enrollment [19,20].
In our experience conducting the clinical trial, we also found that there was an impact of the availability of other evidencebased treatments on the decision making of both patients and providers. Anecdotally, early in the pandemic, when no evidence based treatments were available, providers were more enthusiastic, and this translated into higher patient participation. As more treatment options became available, and early trials about other IL6 receptor therapies became available, attitudes and enthusiasm changed. In addition, some site principal investigators and our own anecdotal discussions with providers suggested that information disseminated on social media impacted their clinical decision making and counseling of patients. At least one patient also reported that he read about the treatment on social media and that was a major driver of his decision not to enroll. Future studies should investigate these factors more formally; as information is increasingly shared through non-traditional settings, these external factors may only grow in importance in the future.
The limitations of this report include the setting, which was conducted in the VISN-1 clinical trials network. Reasons for non-participation in this population may be different than in others, particularly younger populations and populations that include more women. Children have different consent processes and thus findings may not be generalizable to this population in particular. Insufficient data were available to assess the impact of a legally authorized representative versus the patient providing consent; it is possible that factors driving non-participation varied between these two groups. It is also possible attitudes and beliefs about clinical trial participation may vary by region. Limited demographic data that was obtained, particularly in regards to ethnicity which is a factor known to influence clinical trial enrollment, which limited our ability to fully assess some patient-level factors known to impact decisions about clinical trial participation. The amount of demographic and comorbidity data that was obtained changed over the trial period and varied across sites. The changing nature of the pandemic and the availability of other evidence-based interventions may have impacted patient decision making. In addition, early in the study, only patients with more severe disease were eligible; it is possible that disease severity was a factor that drove high enrollment rates early on. Although physician attitudes are known to be an important factor in patient decision making about clinical trials, these were not assessed in this study, and thus only patient perceptions of provider attitudes are reflected [21].
Increasing enrollment into COVID-19 therapeutics trials and therapeutic trials more broadly remains of highest priority though patient enrollment remains one of the biggest barriers for conducting adequately randomized and powered trials [4,5]. To our knowledge, this is the first attempt to qualitatively identify barriers to COVID-19 therapeutic trial participation. Understanding the reason and attitudes behind declining to enroll may help investigators address them during the recruitment and consenting process and thereby increase enrollment and retention rates in future studies.
This material is the result of work supported with resources and the use of facilities the VISN-1 clinical trials network and the VA Boston healthcare system. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
WBE, PM, and JMS were site investigators for a study funded by Gilead Sciences (funds to institution). WBE was supported by NIH NHLBI1K12HL138049-01 during the conduct of the clinical trial and reports funding from the VA Health Services Research and Development Service. All other authors report no conflicts of interest to report.
The VISN-1 Clinical Trials Network COVID-19 investigators includes Gheorghe Doros, PhD; Judith Strymish, MD; Rupak Datta, MD, PhD; Rekha Goswami, MD; Matthew Jankowich, MD; Nishant Shah, MD, MPH; Thomas Taylor, MD; Sara Page, MPH; Cynthia Hau, MPH, and Rupali Dhond, PhD.
This clinical trial would not have been possible without the support of the entire inpatient medical staff at the participating sites for their tireless efforts helping the study team identify and screen patients. We would also like to thank the VISN-1 Clinical Trials Network and Dr. William Boden for his support of the trial, the VA Boston Research Pharmacy and Drs. Antoun Houranieh and Jane Hughes for all of their efforts procuring and distributing study medication. We would also like to acknowledge the help and support of Drs. Michael Charness, Lisa Soleymani Lehmann, Carole Palumbo, and David Thornton, as well as the research efforts of Rebecca Anderson, David Ardito, Karen Evans, Jodi Okrant, and Patricia Spencer.
The views expressed in this manuscript are those of the authors and they do not necessarily reflect the views of the United States federal government or the department of veteran’s affairs.
Citation: Dassum SR, Ferguson R, Woods P, Flynn M, Visnaw K, Holmberg E, et al. (2022) Patient-Reported Reasons for COVID-19 Therapeutics Clinical Trial: Findings from a Multi-Center Investigation. J Clin Trials. 12:508.
Received: 10-Jun-2022, Manuscript No. JCTR-22-17890; Editor assigned: 13-Jun-2022, Pre QC No. JCTR-22-17890(PQ); Reviewed: 17-Jun-2022, QC No. JCTR-22-17890; Revised: 15-Sep-2022, Manuscript No. JCTR-22-17890(R); Published: 23-Sep-2022 , DOI: 10.35248/2167-0870.22.12.508
Copyright: © 2022 Dassum SR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.