Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2013) Volume 4, Issue 1
Oxidative steam reforming of ethanol for hydrogen production was studied over 1%Rh/CeO2Al2O3 catalyst prepared in the laboratory. Support with CeO2 content of 5wt% on γ -Al2O3 was prepared by impregnation with Ce(NO3)3.6H2O solution. The effects of temperature (450°C and 550°C), Water/Ethanol ratio (mol/mol) (3 and 8) and Oxygen/Ethanol molar ratio (0.23 and 0.3) were studied at 1 atmospheric pressure in a down-flow tubular fixed bed reactor. The percentage conversion of ethanol ranged from 90% to 98% and maximum hydrogen selectivity was observed up to 70% at a selected Water /Oxygen/Ethanol molar ratio at 550°C. On increasing Water/Ethanol molar ratios in the feed, the catalytic activity for hydrogen production could be enhanced.
Keywords: Hydrogen; Bioethanol; Oxidative steam reforming; Catalyst
The present renewable energy sources like solar, wind, tidal, biomass energy being utilized are site specific, intermittent and thus not stable. Hydrogen has been identified as a potential alternative fuel produced from renewable energy sources. It has the highest energy content per unit weight, 120 KJ/g [1] and is the only carbonfree fuel which ultimately oxidizes to water as a combustion product. Therefore burning hydrogen has the potential to meet a wide variety of applications and also it does not contribute to greenhouse emissions [2]. Hydrogen can be used either as a fuel for direct combustion in an internal combustion engine or as the fuel for a polymer electrolyte membrane (PEM) fuel cell [3,4]. Thus, hydrogen has many social, economic and environmental benefits and has the long-term potential to reduce the dependence on fossil fuel and lower emissions from the transportation sector.
Hydrogen can be produced from variety of feed stocks and among them, biomass is being considered as a promising source of hydrogen. Hydrogen can be produced from organic waste and waste water by anaerobic fermentation [5]. Bioethanol is of great interest as a feedstock for hydrogen production due to its renewability and low toxicity. Bio-oil produced from biomass has a complex composition with more than 200 different compounds. These includes mainly acids, alcohols, glycerol, aldehydes, ketones and lignin derived oligomers [6-9]. Biooil can be produced in large amounts from biomass such as agricultural wastes and forestry residues and hence is a renewable resource, as against methanol and gasoline. Ethyl alcohol is preferable as the hydrogen resources since it can be easily produced by degradation and fermentation of biomass. It can be produced renewably from several biomass sources (sugarcane, beet root, corn, etc.) including waste materials from agro-industries or forestry residue materials, organic fraction of municipal solid [10]. The ethanol obtained in this way is named bio-ethanol, a mixture of ethanol and water (18-23 wt% ethanol). Hydrogen can be produced through different methods like water electrolysis, gasification, partial oxidation reactions of heavy oil, and steam-reforming reactions. The most effective process for hydrogen production from ethyl alcohol are - the steam reforming reactions, partial oxidation and oxidative steam reforming reactions.
The steam reforming reaction of ethanol is endothermic and produces CO2, a global warming gas, as a byproduct.
C2H5OH + 3H2O → 6H2 + 2CO2 ΔH25°C = 41.6 kcal/mol (1)
In partial oxidation steam is replaced by oxygen and hydrogen is produced by exothermic reaction.
C2H5OH + 3/2O2 → 3H2 + 2CO2 ΔH25°C = -132.0 kcal/mol (2)
In oxidative steam reforming ethanol can be reformed by cofeeding steam and oxygen.
C2H5OH + (3-2δ) H2O + δ O2 → (6-2δ) H2 +2CO2 ΔH25°C ≈ 0 kcal/mol (3)
This process can be made auto thermal when δ=0.35. This does not require any external heat to be supplied as the thermal energy generated in the partial oxidation of ethanol is used for steam reforming. Thus it is also called Auto thermal steam reforming [11,12].
The development of optimal catalytic materials for the steam reforming of ethanol is an important issue. Selecting a suitable support and addition of a proper metal to the metallic active phase are two important factors which play a crucial role in enhancing hydrogen production and ethanol conversion.
Rh has been shown to have excellent ability to break C-C bond and increased selectivity of C1 products (CO, CO2, CH4). The main products are CO and CO2 which indicates that all the H atoms of ethanol can participate in hydrogen production giving highest 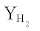 [13,14]. This capability is related to its high-lying d-band structure and with empty d states, which lowers the C-C bond dissociation barrier by stabilizing the intermediates [15,16]. Among the noble metals on various supports, Rh was found to be the most active metal (Rh, Pd, and Pt) with alumina as a support in the ethanol steam-reforming reactions [17]. Rh, Ru and Ir on Al2O3 support have been shown to have highest CH4, CO and CO2 selectivity and best value of
[13,14]. This capability is related to its high-lying d-band structure and with empty d states, which lowers the C-C bond dissociation barrier by stabilizing the intermediates [15,16]. Among the noble metals on various supports, Rh was found to be the most active metal (Rh, Pd, and Pt) with alumina as a support in the ethanol steam-reforming reactions [17]. Rh, Ru and Ir on Al2O3 support have been shown to have highest CH4, CO and CO2 selectivity and best value of 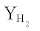 compared to other metals [16]. However, the high cost of noble metals is a major limiting factor in their use for hydrogen production via steam reforming.
compared to other metals [16]. However, the high cost of noble metals is a major limiting factor in their use for hydrogen production via steam reforming.
Al2O3 was shown to convert 100% ethanol at temperature of 350°C which was related to its large adsorption capacity [18,19]. But the yield of hydrogen (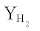 ) was very small was attributed to C2H5OH dehydration to C2H4. Al2O3 is an active catalyst but it has acidic sites on its surface which promotes coke formation by polymerization of
) was very small was attributed to C2H5OH dehydration to C2H4. Al2O3 is an active catalyst but it has acidic sites on its surface which promotes coke formation by polymerization of 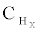 species. Cavallaro et al. [20] reported that Rh is active metal and it reduces coke formation. Rh/Al2O3 was found to be more active than Pd/Al2O3, Ni/Al2O3, or Pt/Al2O3 as reported by Breen et al. [17]. Compared to Al2O3 low surface area supports are preferred like ceria, zirconia is better because of their preferred thermal stability during steam reforming. Rh/CeO2 showed almost 100% activity and produced 5 mol H2 in oxidative steam reforming at 300-800°C [21]. At high Rh loading, Rh/Al2O3 was promising for C2H5OH steam reforming. H2 yield was 5.5 mol [20,22]. Rhodium catalyst derived from a chlorinated metal precursor was found be effective [23].
species. Cavallaro et al. [20] reported that Rh is active metal and it reduces coke formation. Rh/Al2O3 was found to be more active than Pd/Al2O3, Ni/Al2O3, or Pt/Al2O3 as reported by Breen et al. [17]. Compared to Al2O3 low surface area supports are preferred like ceria, zirconia is better because of their preferred thermal stability during steam reforming. Rh/CeO2 showed almost 100% activity and produced 5 mol H2 in oxidative steam reforming at 300-800°C [21]. At high Rh loading, Rh/Al2O3 was promising for C2H5OH steam reforming. H2 yield was 5.5 mol [20,22]. Rhodium catalyst derived from a chlorinated metal precursor was found be effective [23].
Based on literature it can be revealed that ethanol conversion and hydrogen selectivity strongly depends on the type of metal and support chosen as catalyst materials, preparation methods, and operating conditions for reforming reaction such as water/oxygen/ ethanol molar ratios, space time and temperature. Incorporation of oxygen in the steam reforming reaction lowers the temperature required for the reforming reaction. However, limited data are available on the choice of a suitable catalyst and reactions for the oxidative steam reforming of ethanol. Therefore this study aims at studying the catalytic oxidative steam-reforming of ethanol for the production of hydrogen using 1%Rh/5%CeO2 Al2O3 catalyst along with quantitative determinations of hydrogen and hydrocarbons.
Catalyst preparation and characterization
Support with CeO2 content of 5wt% on γ -Al2O3 was prepared by impregnation with Ce(NO3)3.6H2O solution. After impregnation, samples was dried overnight at 110 ± 5°C in oven and subsequently calcined at 550 ± 10°C for 5 h. The supported Rh catalysts with Rh loading of 1 wt% were prepared by the incipient impregnation method, using RhCl3.xH2O (40%). The catalyst thus prepared was dried overnight at 110 ± 5°C in oven and subsequently calcined at 550 ± 10°C for 5 h. It was further powdered and pelletized (3 mm size) using an automatic palletizing press (Techno Search AP-15) and subsequently crushed and sieved to an average particle size of 0.6 mm.
The BET surface area, total pore volume and pore size distribution of the catalyst was determined from nitrogen adsorption/desorption isotherms measured at -196°C using Micromeritics ASAP 2010 apparatus.
Catalytic activity measurements
Before testing, the catalyst was reduced under flowing hydrogen (30 ml min-1) at 550°C for 5 hr using N2 as carrier gas (30 ml min-1). Reactivity testing was carried out at atmospheric pressure in a packed bed tubular reactor (down flow) for 5 hr each. 3 mg catalyst diluted in 1 mg crushed ceramic beads was used for each test. Peristaltic pump was installed as feed pump to supply the feed (mixture of ethanol and de ionized water) from glass storage tank to pre heater. The schematic diagram and other details of the experimental setup are given elsewhere [24]. The capacity of peristaltic pump was maintained in the range of 0.4 ml/min. In order to carry out reaction in vapor phase, the water-ethanol solution was fed to the reactor using peristaltic pump through vaporizer where it was converted into the vapor at 200°C. The catalyst reactivity was tested for different conditions like H2O/Ethanol (W/E) molar ratio 3 and 8, O2/Ethanol (O/E) molar ratio 0.3 and 0.23, temperatures 450°C and 550°C.
All the gaseous products H2, O2, N2, CO, CH4, CO2 were analyzed using a gas chromatograph (Model Nucon 5700) operated under TCD mode. The unit was equipped with molecular sieve column, and Argon (30 ml/min-1) was used as carrier gas. The column conditions were as follows: Injector Temperature: 110°C, Oven Temperature: 60°C, Detector temperature: 120°C, TCD Current: 117 mA.
The condensate volume was used to approximate the ethanol conversion. The liquid product analysis was done using Nucon 5700 gas chromatograph and operated in FID mode. The column conditions were as follows: Column: SS column (3.2 mm OD × 1.8 m long) packed with carbosphere (80-100 mesh), Carrier gas: Nitrogen (30 ml/min), Injector temperature: 210°C, Oven temperature: 80°C, Detector temperature: 230°C. The chromatograms were recorded and peak areas were calculated using Winacds 6.2 software. The carbon /hydrogen balance for all the runs was checked and runs where mass balance was above 95% were considered for the data analysis. Some runs were also carried out in duplicate for the confirmation of data.
The hydrogen yield and selectivity of species (P=H2, CO, CO2, CH4) were calculated as follows:
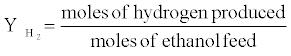
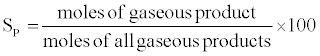

The BET surface area, pore volume and pore diameter of 1%Rh/5%CeO2 Al2O3 catalyst was 203.7 m2/g, 0.6 cm3/g and 108.5 A° respectively. The decrease in the surface area of catalyst is probably due to the interaction between Al2O3 and CeO2, and a partial blockage of the smaller pores in alumina by oxide additive. Damyanova et al. [25] reported BET surface area and pore volume of Al2O3 support as 205 m2/g and 0.5 cm3/g respectively at 500°C. The low ceria loading is also expected to stabilize alumina against surface area loss by preventing the transformation of γ-Al2O3 to α-Al2O3, which depends on the CeO2 loading [25-27].
Prior to the experimental analysis, thermodynamic analysis of oxidative ethanol steam reforming was also carried out to understand the viability of reaction-product and to develop relationships between process variables (i.e., temperature, pressure, and feed composition) and the product distribution. Based on these results catalytic performance was investigated at limited set of conditions. The thermodynamic results are briefly presented in figure 1. Results of thermodynamic study revealed that higher water – to – ethanol ratio favours the hydrogen production as it promotes water gas shift reaction activity. However very high water content also increases the reactor loading therefore optimal water to ethanol ratio is desired. Also higher oxygen in feed reduces the hydrogen selectivity as part of hydrogen consumes during the process. Therefore further experiments were carried out only at selected conditions.
Catalytic activity
The product obtained from steam reforming was a gas mixture consisting of H2, CO, CO2 and CH4 with no acetaldehyde or C2 products. This result was comparable to Ni/Al2O3 catalyst supported on CeO2, ZrO2and CeO2–ZrO2 [28] and suggests that ethanol decomposition and steam reforming are the main reactions in the reactor,
C2H5OH →CO + CH4 + H2 (4)
C2H5OH + H2O→ 2H2 + CO2 + CH4 (5)
In the presence of oxygen, due to the oxidation of ethanol and hydrocarbon products, as well as the Boudaurd reaction and water formation can take place.
C2H5OH + O2→CO2 + CO + 3H2 (6)
2CH4 + 3/2O2 = CO2 + CO + 4H2 (7)
CO + 1/2O2 = CO2 (8)
H2 + 1/2O2 = H2O (9)
2CO → CO2 + C (10)
Effect of Water/Ethanol ratio
The bio ethanol is a dilute aqueous solution of ethanol (18-23 wt% ethanol). The high amount of water helps in suppressing coke formation, favoring water gas shift reaction during ethanol steam reforming reaction but also demands need of high enthalpy to evaporate water. Figures 2a and 2b show the effect of water on H2 selectivity at temperature 550°C. Almost all the ethanol converted during the reaction at these conditions. It is evident that the H2 selectivity increases with increasing water content for a given temperature. The high amount of water helps in promoting complete ethanol steam reforming reaction and also helps in water gas shift reaction.
The selectivity of SCO, S 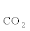 and S
and S 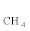 are expected to increase due to H2O-assisted C-C bond cleavage. However, only S
are expected to increase due to H2O-assisted C-C bond cleavage. However, only S 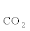 follows this trend in the experimental observation and this contradiction corresponds to that steam plays an important role in the WGSR and the steam reforming of methane (SRM) for CO and CH4, respectively [16].
follows this trend in the experimental observation and this contradiction corresponds to that steam plays an important role in the WGSR and the steam reforming of methane (SRM) for CO and CH4, respectively [16].
The CO selectivity decreases for increasing water content in feed as water gas shift reaction is promoted in forward direction. For the same reason the CO2 selectivity increases with addition of water in feed. The CH4 selectivity decreases with increasing water content in feed as methane reforming reaction is promoted.
CO + H2O → CO2 + H2 (11)
CH4 + H2O → CO + H2 (12)
Both the reactions support the experimental observations that S 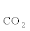 increases from 19.9% to 20.9% as the W/E ratio increases from 3 to 8 at 550°C. The decrease for SCO from 9.5% to 7.8% indicates that the rate of H2O-assisted C-C bond cleavage becomes slower than that of WGSR at higher W/E ratios. The variance of S4CH(15.6% to 6.1%) indicates that the SRM reaction is faster than H2O assisted C-C bond cleavage. In addition, both WGSR and SRM can further enhance the production of H2, as expressed in Reactions (11) and (12). This result explains the dramatic increase of S
increases from 19.9% to 20.9% as the W/E ratio increases from 3 to 8 at 550°C. The decrease for SCO from 9.5% to 7.8% indicates that the rate of H2O-assisted C-C bond cleavage becomes slower than that of WGSR at higher W/E ratios. The variance of S4CH(15.6% to 6.1%) indicates that the SRM reaction is faster than H2O assisted C-C bond cleavage. In addition, both WGSR and SRM can further enhance the production of H2, as expressed in Reactions (11) and (12). This result explains the dramatic increase of S 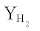 from 55.0% to 65.2%.
from 55.0% to 65.2%.
Effect of Oxygen/Ethanol (O/E) ratio
The effect of O/E ratios was examined at two different O/E 0.23 and 0.3 on 1% Rh- 5%CeO2 Al2O3 at a W/E=8 at 550°C. Oxygen, similar to steam, can quickly form atomic O and react with ethanol and its fragments. Thus, the catalytic behavior varies with O/E ratios (Figure 3). 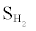 increases as the O/E ratios increases. For example,
increases as the O/E ratios increases. For example, 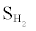 rises slightly from 65.2% to 65.3% as the O/E ratio increases from 0.23 to 0.3 at 550°C. The improvement in
rises slightly from 65.2% to 65.3% as the O/E ratio increases from 0.23 to 0.3 at 550°C. The improvement in 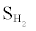 resulting from a higher O2 concentration follows different mechanisms than that obtained with higher W/E ratios. At higher O/E ratios, additional H2 will not be produced from WGSR and SRM. Instead, CO and CH4 will follow the oxidation processes in reactions (13) and (14), respectively.
resulting from a higher O2 concentration follows different mechanisms than that obtained with higher W/E ratios. At higher O/E ratios, additional H2 will not be produced from WGSR and SRM. Instead, CO and CH4 will follow the oxidation processes in reactions (13) and (14), respectively.
Thus, the slight improvement in 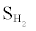 from 65.2% to 65.3% as the O/E ratio increases from 0.23 to 0.3 at 550°C corresponds to the enhancement of the breaking of the C-C bond of ethanol. Thus all selectivity, S
from 65.2% to 65.3% as the O/E ratio increases from 0.23 to 0.3 at 550°C corresponds to the enhancement of the breaking of the C-C bond of ethanol. Thus all selectivity, S 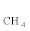 , SCO and S
, SCO and S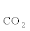 increase as the O2/ethanol ratio increases. The S
increase as the O2/ethanol ratio increases. The S 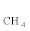 and SCO increase significantly from 5.8% and 6.1% to 7.1% and 7.8%, respectively whereas, S
and SCO increase significantly from 5.8% and 6.1% to 7.1% and 7.8%, respectively whereas, S 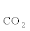 decreased from 22.0% to 20.9% with an increase in O2/Ethanol. In O2-assisted OSR, the production and consumption of CH4 and CO correspond to the rates of ethanol C-C bond cleavage and oxidation processes in reactions.
decreased from 22.0% to 20.9% with an increase in O2/Ethanol. In O2-assisted OSR, the production and consumption of CH4 and CO correspond to the rates of ethanol C-C bond cleavage and oxidation processes in reactions.
CO + 1/2O2 → CO2 (13)
CH4 + O2 → CO2 + 3 H2O (14)
Thus, the observed increase in S 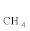 and SCO imply that O2 can efficiently break the C-C bond to produce more C1 products, but the resulting CH4 and CO get oxidized to CO2 at relatively lower rates. This shows the lower oxidation rates of CO and CH4 over the Rh based catalyst.
and SCO imply that O2 can efficiently break the C-C bond to produce more C1 products, but the resulting CH4 and CO get oxidized to CO2 at relatively lower rates. This shows the lower oxidation rates of CO and CH4 over the Rh based catalyst.
Effect of temperature
Effect of temperature can be seen by comparing the results of figures 2a and 4. The selectivity of H2 increased from 61.5% to 65.3% while that of CH4 decreased from 10.0% to 6.1% with increasing temperature from 450 to 550°C. Meng et al. [29] reported that for Rh supported catalysts methanation reaction occurs at high temperatures. The increase of 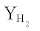 directly corresponds to the increase of ethanol conversion which corresponds to kinetic rates [16]. The extent of methanation reaction which also accounts for generation of methane by hydrogenation of COx compounds decreases with increasing temperature due to exothermic nature of reaction. Thus selectivity of CO2 decreases from 21.6% to 20.9% while that of CO increases from 6.9% to 7.8% at higher temperatures because water gas shift reaction begins to shift in backward direction as it is an exothermic reaction, especially around 550°C. However, further experiments are in progress at different temperatures to investigate the mechanism.
directly corresponds to the increase of ethanol conversion which corresponds to kinetic rates [16]. The extent of methanation reaction which also accounts for generation of methane by hydrogenation of COx compounds decreases with increasing temperature due to exothermic nature of reaction. Thus selectivity of CO2 decreases from 21.6% to 20.9% while that of CO increases from 6.9% to 7.8% at higher temperatures because water gas shift reaction begins to shift in backward direction as it is an exothermic reaction, especially around 550°C. However, further experiments are in progress at different temperatures to investigate the mechanism.
The observed ethanol conversion was in the range of 90-98% at all investigated conditions. Breen et al. [17] reported nearly complete conversion of ethanol and approx 70% S 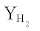 using 1% Rh/Al2O3 and 1% Rh/CeO2-ZrO2. Similarly Frusteri et al. [30] and Erdohelyi et al. [31] also observed more than 90% conversion of ethanol with 91% and 82% S
using 1% Rh/Al2O3 and 1% Rh/CeO2-ZrO2. Similarly Frusteri et al. [30] and Erdohelyi et al. [31] also observed more than 90% conversion of ethanol with 91% and 82% S 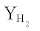 respectively using 3% Rh/MgO and 1% Rh/CeO2 catalysts.
respectively using 3% Rh/MgO and 1% Rh/CeO2 catalysts.
1% Rh/CeO2-ZrO2 catalyst was found effective for the oxidative steam reforming of bioethanol. Results revealed that addition of oxygen to the ethanol water stream is crucial in improving the activity and stability of the catalyst system. The maximum H2 yield of 4.2 was obtained out of 6 at 550°C at 1 atm. pressure, molar ratio water to ethanol molar ratio 8:1 and oxygen to ethanol ratio 0.3:1. The H2 yield increases from 2.0 to 4.2 when temperature was increased from 450°C to 550°C. Also ethanol conversion increased from 90 to 98% when water to ethanol ratio of the feed was changed from 8:1 to 3:1.
One of the Author, Dr. Anamika, is thankful to the Department of Chemical Engineering, IIT Delhi and the Director, Anand Engineering College, Agra for supporting to attend the ‘Summer Faculty Research Fellow Program’ under Continuing Education.