Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2017) Volume 8, Issue 2
The contact angle of some biological liquids was investigated on different biocompatible surfaces and nanoparticles were employed to improve their wetting ability. Biocompatible nanoparticles based on titanium, silver and gold were prepared using the technique of the pulsed laser ablation in liquids. The nanoparticles were characterized in size, shape, coalescence and concentration in solutions. Solid biocompatible substrates based on polyethylene, hydroxyapatite and titanium were employed as simulation of prosthetic surfaces. Measurements of contact angle were performed in different biological solution, including the human blood, with respect to the distilled water, without and with the use of the metallic nanoparticles at concentration of the order of 80-370 μg/ml.
Keywords: Nanoparticles; Laser ablation; Contact angle; Biocompatibility; Interfaces
More and more the nanotechnology is becoming important and promising in different fields of the science. In Medicine the nanotechnology can be applied with success to healthcare and is becoming very revolutionary for medical diagnostics and therapy. Medical imaging using NMR, TAC, PET, X-rays and thermal imaging, thermal and radio therapy, protontherapy and other techniques can be improved using adapt contrast medium based on the new generation of materials using nanoparticles (NPs) [1-3]. Thermal treatments, surface and depth diagnostics, drug delivery, tissue regeneration, adhesion of biomaterials to biological liquids and tissues, represent only some applications of the nanoparticles in nanomedicine [4,5]. Nanoparticles can be used as pure or as functionalized so that their chemical inertia or reactivity can be controlled by the molecules chemically bond to them [6]. Their transportation in the human body though the blood flux, their diffusion due to high concentration gradients in tissues, their uptake in organs and liquids, their introduction in cells and their concentration time decay from tissues plays important roles for the applicability in nanomedicine.
In particular in this paper they are presented as agent to modify the wetting ability of liquids which are deposited on biocompatible surfaces, such as that wetting prosthesis based on polymers, ceramics and metals. The use of permanent prosthesis such as those used in orthopaedic and in dentistry, need of high adherence and high hydrophilic action with the bone from the earliest moments of fixation and the use of resins and cements for the anchorage need to determine adherent interfaces without holes formation between the different materials. The high wetting ability in this case permits the fast cell colonization and adherence to the biomaterial [7]. The use of removable prosthesis, instead, need of surface with high hydrophobic action because must be removed after a period more or less long. Their insertion in the body is only temporary and the prosthesis must provide for their removal without causing collateral damage, as occurs for catheters, contact and intraocular lens and orthopedic temporal fixers. Moreover, the use of many nanoparticles colloidal solutions, such as silver and bismuth, has antibacterial, antifungal, and antiviral properties and can be employed together physiological solutions in living tissues and organs or deposited as thin film on different prosthesis surfaces [8].
The use of solutions containing metallic nanoparticles at different concentrations, modify significantly the original liquid physical and chemical properties, such as the optical, the surface tension, density, viscosity, thermal and electric conduction, vapour pressure, the PH and other parameters [5,9]. The wetting ability of the liquid on a given surface changes introducing specific nanoparticles at the interface. This aspect is of special interest in Biology and Medicine, especially due to the impact on the protein absorption and the cellular response in ambit of biological tissues-biomaterial interface [10]. The contact angle of the liquid in presence of nanoparticles may be altered depending on the surface properties, such as composition, chemical reactivity, roughness, crystalline structure, grain contours, porosity, permeability, etc. [11]. On these last aspects is focused the aim of this article aims to demonstrate as specific metallic nanoparticles may alter the properties of hydrophobic or hydrophilic behaviour of the used materials. The use of some biocompatible nanoparticles species at low concentration in water, physiological solutions and blood, determines a different wetting ability with respect to different substrate surfaces, as it will be presented and discussed in this paper.
Metallic nanoparticles based on pure titanium, silver and gold, were prepared using laser ablation in liquids. A Nd:YAG laser was employed at 1064 nm wavelength, 3 ns pulse duration, 200 mJ pulse energy, 1 mm2 focused spot and 10 Hz repetition rate. The metals, as sheets of about 1 cm2 surface and 1 mm thickness, were placed on the bottom of a glass test tube. A volume of 15 ml of distilled water was added and 1 μg of tensioactive (sodium citrate) was employed as stabilizer in order to avoid fast particle coalescence. The ablation yield, I n terms of removed mass per laser shot, was different for each metal and generally using exposition times of the order of 20 min with repetitive laser shots, a total ablation of about 1-10 μg mass per laser shot was produced in the form of nanoparticles in water [12]. A scheme of the used set-up is reported in Figure 1a. The laser ablation removes the first surface layers of the irradiated target producing plasma in which nanoparticles as nucleated. The shape of the nanoparticles is spherical with a size distribution ranging between 10 and 50 nm with an average diameter of about 20 nm. The used concentrations in water and in physiological liquid PL were: 1.2 mg/15 ml for Ti; 2.3 mg/15 ml for Ag; 5.6 mg/15 ml for Au. Flat and very polished surfaces of pure polyethylene (PE), hydroxyapatite (HA) and titanium were employed as substrates. The polished surface roughness was measured using a surface profiler (Tencor P10). Human dental enamel, prepared by the Dentistry Clinics of Catania University, was used as polished hydroxyapatite substrate. Liquid solutions based on distilled water, saline solutions and blood were used both without and with addiction of metallic nanoparticles. The physiological liquid (PL) is prepared as saline solution (Fresenius Kabi Italia 0.9%) composed of 9 gr NaCl dissolved in 1000 ml of water. Human blood, from a healthy volunteer, was extracted in a laboratory of clinical analyzes and then stored in the original sterile tube (BD Vacutainer K3E) with 5.4 mg ethylenediaminetetraacetic acid (EDTA) at a temperature between 2°-6°C. The liquid solution of EDTA binds calcium ions thus inhibiting the coagulation cascade, preserving the blood [13]. In order to avoid the possible deterioration of blood, the measurements of contact angle were performed within 5 days from donation. Before the experiment, the tube was agitated to homogenise the solution and for a long time, until the room temperature was reached.
Figure 1: Scheme of the experimental apparatus for nanoparticles preparation using laser ablation of metallic targets placed in liquids employing a Nd:Yag laser (a) and scheme of the liquid drop deposited on a solid surface to evaluate the contact angle as resulting of the involved forces at the solid-liquid-gas interfaces (b).
The used nanoparticles were characterized using SEM and TEM microscopies, though optical absorbance measurements in the visible wavelength region and performing X-ray fluorescence (XRF) analysis in order to evince the eventual presence of contaminant elements. SEM microscopy and XRF was performed by using a 20 keV electron beam, produced by a FEI QUANTA - mod. Inspect S microscope, at the Department of Physics Sciences of the Messina University. TEM analyses were performed with a FEI Morgagni 268D microscope equipped with a 1 k × 1 k SiS CCD camera Megaview II, operated at the nominal accelerating voltage of 80 kV. Optical absorbance measurements use a high-sensitivity spectrometer (Horiba-Jobin-Jvon, VS70), a Hg-Ar lamp (Oriel Hg(Ar) mod. 6035) and the software code “Lynear” to acquire 100 spectra per second. The wetting angles were measured using the “sessile drop” method based on the observation of the liquid droplet profile deposited on a cleaned solid surface. The drop is generated depositing 1 μl liquid from a calibrated microsyringe on the horizontal substrate surface. A CCD camera connected to the eyepiece of an optical microscope records the drop-substrate interface image of the substrate cross-section. Dedicated software permits to calculate the contact angle with an error of ± 1°. The contact angle θ depends on the balance of the forces of adhesion and cohesion of the liquid to the substrate in presence of air, as schematized in Figure 1b. If θ is greater than 90° the surface is hydrophobic while if θ is less than 90° the surface is hydrophilic. The contact angle can be calculated by the Young equation [14]:
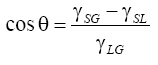 (1)
(1)
where γSG is the surface tension of the solid (in equilibrium with the saturated vapour of the liquid), γSG is the surface tension between solid and liquid, and γLG is the surface tension of the liquid (in equilibrium with its vapour saturated). The wetting ability measurements were performed in three modes: using pure liquids deposited on the different pure substrates; using liquids containing metallic nanoparticles (NPs) on the different substrates; and using liquids deposited on substrates which surfaces were preventively covered by a layer of metallic nanoparticles. This coverage was obtained depositing drops of water, containing little quantity of polyvinyl alcohol (1%) and nanoparticles, on the cleaned substrate surfaces followed by drying process at 50°C for 24 hours. Surface tension τ measurements on the used liquids were performed by the “dropper method” [15] at room temperature and relative humidity (22°C and 35%, respectively), using a 1.2 mm inner radius R glass dropper and an electronic balance (Kern KB 120-3N, max 121 gr, d=0.001 gr) to measure the drop mass M. The surface tension t was determined as average value on 10 measurements (measure error 1%), using the equation:
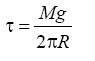 (2)
(2)
where g is the gravitational acceleration.
Figure 2 shows examples of ablation yield evaluations obtained using the above described laser at different fluence (J/cm2) and for different targets placed at 5 mm depth in water. It indicates that the amount of material released to the liquid is a function of the laser fluence and of the nature of the metallic target. The data of ablation yield are affected by an error of about 5%. At the used conditions of 200 mJ pulse energy the real laser energy arriving on the target is about 50% because it is partially absorbed by the liquid just as nanoparticles are produced. Assuming the energy on the target to be 100 mJ per pulse and 1 mm2 spot size, the real laser fluence is about 10 J/cm2, at which the ablation yield is about 0.2 μg/pulse, 3 μg/pulse and 7 μg/pulse for Ti, Ag and Au, respectively. However such yield decreases if laser interact with high nanoparticle concentration in liquids.
By controlling the ablation yield and the mass removed from the target after 10 min irradiation time, for weight difference before and after the laser irradiation, it was possible to produce a mass ablation of 1.2 mg, 2.3 mg and 5.6 mg, for Ti, Ag and Au, respectively, in the form of nanoparticles immersed in 15 ml liquids. SEM and TEM electron microscopy have demonstrated that the nanoparticles are spherical and that their diameter generally is within 10 and 50 nm. The minimum average diameter is found for Ag NPs, of the order of 15 nm, and the maximum for Au, of about 25 nm. Figure 3 shows typical SEM photos of Au (a), Ag (b) and Ti (c) NPs deposited on different substrates and a normalized plot of their diameter distribution (d). A significant effect of NPs coalescence, from nm up to micrometric size, is observed as a function of the NPs permanence time in water and of the solution temperature, in agreement with literature [16]. The prepared NPs have been used immediately, because Van der Walls forces tend to produce NPs aggregation up to enlarge their dimension to micrometric sizes, however the use of surfactant molecules, such as citric acids and others, permits to reduce significantly this unwanted effect [17]. In our experiment the addiction of 1 μg tensioactive has reduced strongly this effect that occurs only for times of the order of 1 week or more.
The substrate roughness represents an important parameter concerning their surface wetting ability, in fact the contact angle decreases with the surface roughness increase, as presented in literature [18]. The used substrates were very polished at sub-micrometric values, so that the influence of the roughness was less as possible. Figure 4 reports three surface roughness, with sub-micrometric values, for the used polyethylene (a), hydroxyapatite (b) and titanium (c) substrates. In all cases the average roughness is sub-micrometric, being of about 0.85 μm in polyethylene, of 0.9 m in hydroxyapatite (dental enamel) and 0.15 μm in titanium. Figure 5 reports three sets of contact angle measurements performed in physiological liquid drops deposited on polyethylene (a), hydroxyapatite (dental enamel) (b) and titanium substrates (c). The initial contact angle is 94°, 53° and 91° for the solution drop on the PE, HA and Ti, respectively, indicating that the polyethylene and the titanium substrates are hydrophobic with respect to the solution at 5.6 mg/15 ml. These angles decrease to minimum value of 80°, 40° and 70° when Au NPs are deposited on the substrate. The reduction of the contact angle is of the order of 10%-20% using AuNPs embedded in the solution and of the order of 15%-25% using the AuNPs only at the solid-liquid interface, in agreement with our previous papers [19].
Figure 5: Liquid drops images of physiological liquid drops deposited on the different polished substrates of polyethylene (PE) (a), hydroxyapatite (HA) (b), and titanium (Ti) (c). The analysis was performed without nanoparticles (left), with NPs deposited at the solid-liquid interface (centre) and using NPs embedded in the solution (right).
In the three cases the measurements were conducted without the use of nanoparticles (left), with the use of Au NPs deposited at the interface (centre) and with Au NPs embedded in the solution (right). Measurements indicate that in all cases the wetting ability increases using the Au NPs, in fact the contact angle decreases due to the action of the NPs. The better wetting ability is obtained using Au NPs at the liquid-solid interface that further decreases the contact angle with respect to the use in the solution. Similar results are obtained also using Ti and Ag NPs embedded in the solution or deposited at the solidliquid interface. The results of the wetting ability analysis using the different NPs are reported, for useful comparison, in the plot of Figure 6 for the different metallic NPs using physiological solution and the three different substrates. Here “No” means liquid without use of NPs, “Int.” means use of NPs only at the solid-liquid interface, and “Liq.” means use of NPs embedded in the liquid.
Although the trend is the same for the different NPs, the contact angle decreasing using Ag NPs is more evident with respect to Au and Ti when these nanoparticles are used as thin film deposited on the substrate than in the liquid. The wetting ability using distilled water and physiological liquid is similar, although the surface tension measurements in water (τ=72 × 10-3 N/m) gives little lower values than in physiological solution (τ=77 × 10-3 N/m), indicating that the cohesion forces in water are less strong than in the saline solution. The measures of surface tension in human blood have indicated an average value of τ=62 × 10-3 N/m, thus a lower cohesion force with respect to the other liquids, indicating that is expected an higher wetting ability of the blood drop with the different substrates. Figure 7 shows three typic al photos at the optical microscope, in air at room temperature ad humidity, of the contact angle of the distilled water (a), physiological liquid PL (b) and human blood drops, without use of any metallic NPs, deposited on the polyethylene substrate. The measured contact angle was 97°, 94° and 76° for water, physiological liquid and blood, respectively.
Figure 8 shows three typical photos at the optical microscope, in air at room temperature ad humidity, of the contact angle of the human blood, without NPs, with the different biocompatible substrates of polyethylene (a), hydroxyapatite (b) and titanium (c) in which the contact angle is 76°, 40° and 56°, respectively. The presence of metallic NPs in water, physiological liquid and blood, determines a percentage decrement of the contact angle that generally is within 10%-20% with respect to the value measured without NPs. The presence of metallic NPs at the solid-liquid interface determines a higher decrement of the contact angle that generally is within 20% and 70% with respect to the value measured without NPs. Experimental data demonstrated that the use of Ag NPs enhance such wetting ability effect when used at the solid-liquid interface more than for the other metallic nanoparticles. Of course, many factors influence the obtained results, from the dimensions of the used nanoparticles to the surface tension of the liquid and to the roughness of the substrate surface that influence drastically the wetting ability effect. Generally, as was demonstrated experimentally, the increment of the surface roughness increases the wetting ability, reducing the contact angle of the liquid drop on the solid surface, and the increment of the surface tension, increasing the cohesion force between the liquid molecules, decreases the wetting ability and increases the contact angle. The Ag NPs average dimensions of the order of 15 nm, little lower than for the other metals, could be enhance such wetting ability effect more than for the other type of NPs. In all cases when metallic nanoparticles are employed both in the solution than as thin film deposited on the solid surface, the contact angle decreases demonstrating an enhancement of the liquid wetting ability.
The measurements of contact angle of biological liquids on biocompatible surfaces permit to evaluate the possible attachment of cells, such as fibroblasts, to the material and to evaluate the possible adhesion and growth of biological tissue on the prosthesis depending by many factors from which that concerning the surface wetting ability. Removable prosthesis, such as catheters, orthodontic wires, contact lens and orthopaedic devices, must have high hydrophobic action to avoid or to reduce the possibility of cell growth on the implanted material. Permanent prosthesis, instead, need to be anchored strongly to the tissue to perform their mechanical, medical and biological role, such as femoral stems and acetabular cups, knee prosthesis, bone fixers, orthopaedic bars, screws, cups and others. In this last case a roughness lower than 1 micron is necessary to maintain low the contact angle and confer high hydrophilic properties permitting a good adhesion to the liquid. This paper demonstrates that the biological liquids, such as water, physiologic solution and blood, transporting living cell in the body, can be made more efficient in wetting ability adding biocompatible metallic NPs at low concentration. The used concentration ranging between 1.2 mg/15 ml for Ti and 5.6 mg/15 ml for Au, in fact, are sufficiently low to be below any toxic level and, due to the high biocompatibility of the used elements and to the nanometric dimensions of the particles, are practically exempt from any complication. The NPs can be transported by the blood flux, can be injected locally, can be chemically functionalized to be transported physiologically to organs and tissues or to be diffused though respiration or ingestion.
Such use enhances the wetting ability up to about 70%-80% as measured for Ag deposited on soli surface as a thin film. The decrement of the contact angle means that the surface tension of the liquid containing NPs is lower and that the attractive forces due to Van der Waals, electrostatic and dipolar, and Laplace pressure occurring between liquid and solid interface increase. Literature reports that using other nanoparticles and other substrates with respect to those used in this study it is possible to find cases of increment of the contact angle, i.e., increment of the hydrophobic character of the system, as occurs in polymethylmethacrylate (PMMA) and polythethrafluoroethylene (PTFE) substrates and with the use of Cu NPs embedded in the solution or deposited n the solid-liquid interface [19].
The used nanoparticles can be employed not only to improve the adhesion of the liquid to the solid surface but also for other secondary effects, such as for the antibacteric, sterilizer and cicatrizing effects induced in the biological environment. NPs can be transported in extra and intra cellular liquids in order to enhance the contrast imaging using X-rays absorption enhancing the contrast medium action. NPs can be also employed to be accumulated in disease tissues, as in tumour tissues, to be prepared to have high absorption due to exposition to ionizing radiation as performed in radiotherapy using X-rays, electrons and protons (protontherapy) [20].
Laser ablation can be used to prepare metallic biocompatible spherical NPs in water with size between 10 and 50 nm.
The contact angle of biological liquids containing the metallic NPs at concentration within 80 μg/ml and 373 μg/ml or in the case of NPs deposited at the solid-liquid interface decreases significantly with respect to the case in which none nanoparticle is used. The effect of nanoparticles improving the wetting ability of biological liquid can be employed in different field and in special manner in ambit of Bio- Medical environment. It can be employed to enhance the anchorage of permanent prosthesis with soft and hard tissues, to improve healing effects, to improve the antibacteric effect of biological surfaces and to sterilizer solid surfaces of prosthesis before their implantation in the body. Moreover the use of functionalized nanoparticles with adapt molecules may permit their transport or their injection in tumor tissues to be better contrasted as X-ray imaging o to be irradiated during radiotherapy to enhance the absorbed dose improving he therapeutic efficiency again the cancer.
This research was supported by University of Messina Research and Mobility 2016 Project (Project code RES_AND_MOB_2016_TORRISI).