Journal of Drug Metabolism & Toxicology
Open Access
ISSN: 2157-7609
ISSN: 2157-7609
Research Article - (2023)Volume 14, Issue 1
Poloxamers (PLs) are commonly used pharmaceutical excipients. According to the different molecular weight, mainly PL124 and PL188 are observed. PL188 with the larger weight, hydrophilic and more application in oral exposure drug delivery systems than PL124. However, the absorption and transport mechanisms of PL188 into intestinal epithelial cells are still unclear. In this study, we study the uptake and efflux of PL124/188 in Caco-2 cells, and subcellular accumulation in Caco-2 cells quantitatived by ultra-high-performance liquid chromatography-triple/time-of-flight mass spectrometry (UHPLC-Q-TOF/MS). The results showed that, the uptake in Caco-2 cells are different: Both PL188 and 124 entered Caco-2 cells through active transport, but PL188 uptake into Caco-2 cells relies on pinocytosis pathway, fosselin-mediated endocytosis pathway, and the endocytosis pathway independent of mestin and fosselin-mediated endocytosis. While PL124 uptake by independent of clathrin- and fossa-mediated endocytosis inhibitors pathway; The effects of PL188 on P-gp protein substrate were different: PL188 was stronger than PL124; There are also differences in subcellular accumulations: PL124 reaches the cell membrane at a slower rate compared to PL188, which rapidly enters the cytoplasm and nucleus, with a higher concentration in the cytoplasm. Both PL124 and PL188 remain in the skeleton. This work provides data basis for our subsequent application of PL188 and PL124 as oral preparations.
Poloxamer; Caco-2; Uptake; Efflux; Subcellular Organelle Accumulation
Poloxamers (PL) are ideal drug delivery carriers because they can be assembled into micelles in a set of solutions at appropriate temperatures and concentrations [1]. According to their molecular weights and ratios of hydrophilic and hydrophobic groups [2]. It contained PL124 and PL188 respectively. The average molecular weight of PL124 is 2090-2360 Da, while that of PL188 is 7680- 9510 Da [3]. They are all used as dispersant, emulsifier, solubilizer, and lubricant [4-8]. However, PL188 is more used in promoting the gastrointestinal absorption enhancers than PL124 [9-12], such as PL188 improving the water solubility of curcumin increases its intestinal epithelial uptake and oral bioavailability [9], which modified florfenicol instant microparticles for improved oral bioavail abilitys [10], and promote products with better solubility, higher stability, superior therapeutic efficacy and less toxicity in treatment of Leishmaniasis as nanoparticles, micellar systerms, lipid nanocarrier, microemulsion and nanoemulsions [11], and also poloxamers can be used as tablet lubricants [12].
Intestinal barrier plays an important role in the efficacy of many oral preparations, gastrointestinal mucosal barrier [13]. The absorption, accumulation and efflux of the gastrointestinal mucosal absorption process play an important role in regulating drug efficacy, including the speed of drug absorption into the blood [14], the degree of drug absorption [15], the transformation form [16], and even the binding effect with the protein crown [17]. Usually, Caco-2 cell model were used to evaluate the absorption and availability of oral drugs through intestinal mucosa [18], and the same model was adopted in this project. In addition, the level of sub organelle accumulation may play an important role in affecting the play of some functional enzymes, for example, nucleation may be involved in regulating the function of some functional proteins. This is especially important in the treatment of intestinal diseases [19]. Therefore, it is necessary to compare the action processes of different molecular weights of PL (124 and 188) in intestinal mucosal cells for absorption, accumulation and expulsion into blood.
In this study, the pathway of uptake and efflux of PL124/188 in Caco-2 cells are investigated by adding inhibitors and subcellular protein extraction kit method, and also using Caco-2 cell, and subcellular accumulation level in Caco-2 cells and quantitatived by ultrahigh-performance liquid chromatography combined with ultra-high-performance liquid chromatography-triple/time-of-flight mass spectrometry (UHPLC-Q-TOF/MS) with an MSALL [20- 22], which have high sensitivity, selectivity and reproducibility.
This study clarifies the uptake and transshipment mechanisms of PL124/188 in intestinal epithelial cells. It provides an explanation for clarifying the mechanism of Poloxam with different molecular weight as gastrointestinal absorption promoter.
Experimental Instruments and reagents
PL124/188 (purity ≥ 99.9%) (MREDA Technology Co., Ltd., Beijing, China); Simvastatin (purity ≥ 99%, Sigma –Aldrich, St. Louis, Missouri, USA); Formic acid (Beijing Chemical Plant, Beijing, China); Genistein (GB10425 ≥ 97%, Shanghai Macklin Company); Chlorpromazine hydrochloride (C834105 ≥ 98%, Shanghai Macklin Company); Colchicine (C804812 ≥ 98%, Shanghai Macklin Company); Quercetin (Q817162, ≥ 97%, Shanghai Macklin Company); VER (BD233406 ≥ 98%, Pedder Medicine); Rho123(R817327, ≥ 99%, Shanghai Macklin Company); Caco-2 cells (Wuhan Prosei Life Technology Co., Ltd.); Dulbecco's Modified Eagle Medium(DMEM) culture medium(WISENT ING); Nonessential amino acids (Shenggong Bioengineering, Co., Ltd.); Fetal Bovine Serum (FBS); Penicillin-streptomycin(PS), HEPES (Shengong Bioengineering (Shanghai) Co., LTD); Cell Counting Kit-8 (Beijing Labgic Technology Co., Ltd.); Subcellular structure protein extraction kit (C500073-0050, Shenggong Bioengineering, Shanghai, Co., Ltd.); BCA protein concentration determination kit (P0012, Biyuntian Biotechnology Company).
Basing on the quantitative method of UHPLC-Q-TOF/MS) with an MSALL to detect PL124 and 188 on Caco-2 cells
Instrumentations and conditions: The samples were qualitatively and quantitatively analyzed by UHPLC-Q-TOF/MS with an MSALL-based method, which are based on our established methods in vivo [20-22]. MSALL scan mode was used to scan the ions. The scan range was 50–1250 Da, and the characteristic fragment ion mass extraction window (MEW) was ± 0.005 Da. Analyst 1.7.1 software (AB Sciex, USA) was used to process the data. Peakview 2.2 software (AB Sciex, USA) was used to optimize MEW. Here, m/z 133.0859, which was composed of three PEO units, was chosen for the quantification of PL188/124, and MEW was set from m/z 133.08 to 133.09.
Preparation of stock solutions, working solutions and QC solutions
Stock solutions (1.0 mg/mL) of PL124/188 and simvastatin (IS) were prepared separately in an acetonitrile-water solution (2/3, v/v). The PL124/188 stock solutions were diluted with acetonitrile– water (2:3, v/v) to 100, 250, 500,1000, 2000, 5000 and 10000 ng/ mL. The IS stock solution was diluted with acetonitrile-water (2:3, v/v) to 5μg/mL. All these solutions were maintained at -4°C before use.
Sample preparation
A 50 μL Caco-2 cell sample (106), 20 μL of the IS solution (simvastatin, 5 μg/mL) and 150 μL of ice-cold acetonitrile were mixed, vortexed for 1 min and centrifuged at 13000 rpm for 5 min to precipitate protein. Forty microliters of the supernatant was injected into a UHPLC-Q-TOF/MS system.
Caco-2 cell culture
The Caco-2 cells were cultured by DMEM medium with 10% FBS, 1% PS and 1% nonessential amino acids, using Petri dish cultured in an incubator with 37°C and 5% CO2.
Caco-2 cell viability analysis
Adherent Caco-2 cells in the logarithmic growth phase were seeded in a 96-well plate. Then, after incubation for 24 h, when the adherent density of the cells was 80%, PL124/188 was mixed with DMEM at concentrations of 1, 5, 10, 100 and 200 μg/mL, and 0.2 mL of the mixture at each concentration was added to 6 parallel wells and incubated for 48 h. The cell viability was detected using the CCK Kit. The OD values at 450 and 600 nm were detected using a microplate reader.
Uptake pathway analysis
Adherent Caco-2 cells in the logarithmic growth stage were inoculated into 6-well plates. After the cell density of each well reached 80%, cell uptake experiment was carried out:
Uptake tests at 4μ and 37μ: The PL124/188 at the concentrations of 10, 50, 100, 500 and 1000 μg/mL was added into the 6-well plates adherent with Caco-2 cells incubation for 2 hours at 4/37°C [23], 4 parallels in each group. Then the cells were washed with ice-cold PBS 3 times, and scraped cell added with 1 mL ultrapure water, then the collected cell suspension was ultrasonic crushing at 100 W and 10 s intermittent 10 times. They were divided into two parts after centrifugation at 1000 r/min for 3 min. One was used to determine the concentration of PL124/188 by UHPLC- Q-TOF/MS with the MSALL-based method, and the other was used to determine the protein content in the samples using a BCA kit. Then, calculating the concentration of PL124/188 at average protein concentration, drawn the concentration-time curve.
Ingestion route analysis: Added PL124/188 (100 μM) to 6-well plates adherent with Caco-2 cells incubation for 30 min, and treated with four cell uptake inhibitors [24]: Colchicine (40 μg/ mL), chlorpromazine (10 μg/mL), genistein (50 μg/mL), and quercetin (100 μg/mL) in DMEM incubation for 2 h, 4 parallels in each group. The next steps were the same as uptake tests.
Efflux analysis and effect on Pgp protein
The concentration of PL124/188 in Caco-2 cells and the effect of Pgp protein substrate Rho123 were analyzed [25]. Per treated with PL124/188 (10, 50 and 100 μM) into the Caco-2 cells pre-walled into 6-well plates for 0.5 h with 4 parallels in each group, then add Rho123 (4 μM) (positive drug) and VER (200 μM), which was followed by incubation for 2 h. The collected cell samples were scraped with 1 mL of ultrapure by a cell scraper in an ice bath, and centrifuged at 9000 g at 4°C for 30 minutes after washed 3 times with ice-cold PBS. Rho 123 content was detected by Enzyme marker at an excitation wavelength of 485 nm and an emission wavelength of 530 nm, PL124/188 concentrations in cell suspension and supernatant were analysed by UHPLC-Q-TOF/MS, protein concentration in the sample were detected with a BCA kit.
Subcellular organelle level accumulation analysis
PL124/188 in subcellular organelle level accumulation was analyzed. The cytoplasm, membrane, nucleus and skeleton of PL124/188 was extracted by subcellular protein extraction kit [26] after PL124/188(100 μM) treatment of Caco-2 cells by 1, 1.5, 2 and 2.5 h, which were evenly inoculated in 6-well plates and 80% adhesion, 3 parallel groups. Then quantitatively detected the accumulation level of PL124/188 in the subcellular organelles by UHPLC-Q-TOF/MS.
Statistical analysis
Statistical tests were performed using Origin 9.0 software, and p < 0.05 was considered to be significant. All data are presented as the mean ± standard deviation (x ± sd). At test was used to compare means among group.
Quantitative methods for PL124/188
LC–MS/MS conditions: PL124/188 and IS can be detected in positive mode and the quasi-molecular ion are all [M+H]+. Figure S1 shows the main fragment ions of IS (Figure S1A), PL 124 (Figure S1B), and PL 188 (Figure S1C). The strongest fragment ions of IS, PL 124/188 are 199.1467, 133.0856 and 133.0856, respectively. Although the fragment ion of PL124/188 are the same, they have different molecular weights, so they show different signal intensities and retention time. Thus, product ion m/z 199.1476 was selected for quantifying IS, and product ion m/z 133.0856 was selected for quantifying PL124/188.
Calibration curve: The chromatograms results of PL124(100 μM), IS (5μg/mL) of blank group, Lower Limit Of Quantification(LLOQ) and Caco-2 cell group were shown in Figure S2, and that of PL188 (100 μM) and IS (5 μg/mL) were shown in Figure S3. The results shown that the retention times of PL124/188 are 2.62 and 2.48 min. The typical equations for PL124/188 were Y=6.7726e-4x + 0.10023, R2=0.9875, and Y=0.06934x + 0.01920, R2=0.9957. The method is linear in the range of 100 ng/mL to 10 μg/mL, and there is no obvious interference, indicating that the established method has good selectivity.
Caco-2 cells viability: The results of the viability of PL124/188 on Caco-2 cells were shown in (Figure 1). PL124/188 had no effect on the viability of Caco-2 cells with the concentration range of 1-200 μM after incubation for 48 h. It shows that PL124/188 has the potential application in gastrointestinal absorption promotion at the concentration range of 1-200 μM.
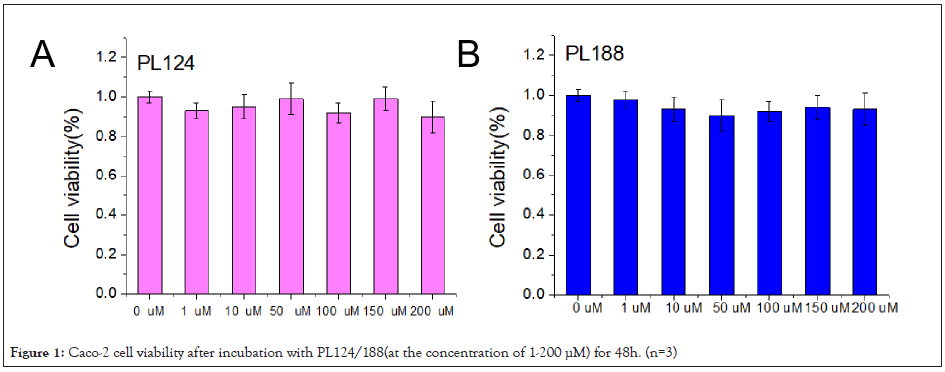
Figure 1: Caco-2 cell viability after incubation with PL124/188(at the concentration of 1-200 μM) for 48h. (n=3)
Uptake pathway on Caco-2 cell
The accumulation concentration-time curves of PL124/188(10- 1000 μM) on Caco-2 cells at 37/4 μ in (Figure 2). These results show a linear relationship between the concentration range-time. PL124 at 37°C, y = 0.7233x + 227.36, R2= 0.9951; at 4°C, y =0.6352x + 98.162, R2= 0.9975. In addition, PL188 at 37°C, y = 0.6123x + 65.66, R2= 0.9998, at 4°C, y = 0.3038x + 20.616, R2= 0.9988. While the uptake concentration of PL124/188 in Caco-2 cell at 37°C > 4°C. It indicates that PL124/188 were uptaked into Caco-2 by an active transportation way.
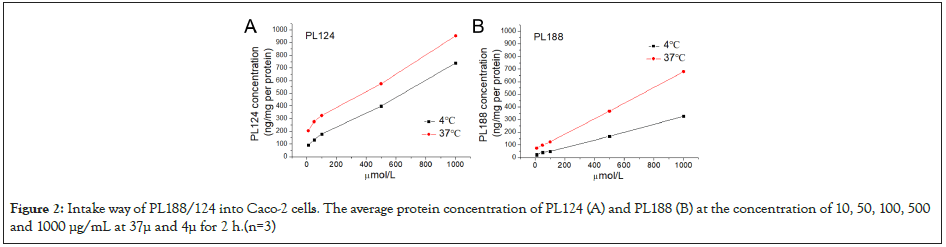
Figure 2: Intake way of PL188/124 into Caco-2 cells. The average protein concentration of PL124 (A) and PL188 (B) at the concentration of 10, 50, 100, 500 and 1000 μg/mL at 37μ and 4μ for 2 h.(n=3)
Further, the specific intake mode of PL124 and PL188 into Caco-2 cells was investigated by adding four different inhibitors. The results were shown in (Figure 3).Compare with the PL124, the average protein concentration of PL188 in Caco-2 was lower after incubation for 2 h (Figure 1). The intake of PL124 by Caco- 2 decreased significantly after add quercetin (P<0.01), while increased after add chlorpromazine, genistein and colchicine (P< 0.01) (Figure 3A). The intake of PL188 by Caco-2 decreased significantly after adding the quercetin, genistein and colchicine (P< 0.01), while no change after adding chlorpromazine (P>0.05) in (Figure 3B).
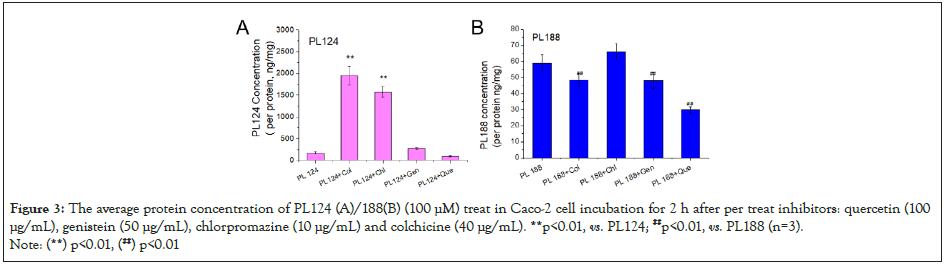
Figure 3: The average protein concentration of PL124 (A)/188(B) (100 μM) treat in Caco-2 cell incubation for 2 h after per treat inhibitors: quercetin (100 μg/mL), genistein (50 μg/mL), chlorpromazine (10 μg/mL) and colchicine (40 μg/mL). **p<0.01, vs. PL124; ##p<0.01, vs. PL188 (n=3).
Note: (**) p<0.01, (##) p<0.01
External discharge on Caco-2 cell
The concentration of Pgp protein substrate Rho123 of PL124/188 on Caco-2 cells after incubation for 4 h. The results are shown in (Figure 4). Compared with the blank group, the positive drug VER significantly increased the secretory concentration of Rho123 (P < 0.01), while PL124/188 (10, 50 and 100 μM) also significantly more increased the concentration of Rho123 (P < 0.01) than VER with concentration- dependent (P < 0.01). The results showed that the effect of PL188/124 on protein substrate was enhanced. Compared with PL124, PL188 increased the concentration of Rho123 more significantly. This indicated that the efflux of PL188 from Caco-2 cells was weaker than that of PL124.
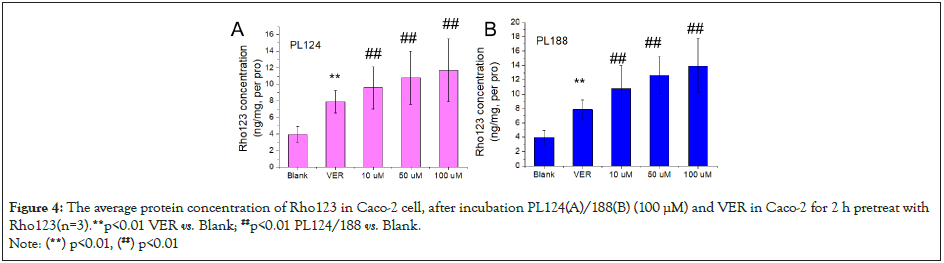
Figure 4: The average protein concentration of Rho123 in Caco-2 cell, after incubation PL124(A)/188(B) (100 μM) and VER in Caco-2 for 2 h pretreat with Rho123(n=3).**p<0.01 VER vs. Blank; ##p<0.01 PL124/188 vs. Blank.
Note: (**) p<0.01, (##) p<0.01
Suborganelle accumulation in Caco-2 cell
The result of suborganelle accumulation of PL124/188 in Caco-2 cell were shown in (Figure 5). The results showed that in the cell membrane: Both of them peaked at 1.5h, but PL124 was slightly higher than PL188 (Figure 5A); in the cell cytosol: Both PL124 and PL188 reached their peak at 1 h and showed a time-dependent reduction, but the concentration of PL188 was higher than that of PL124 (Figure 5B); in the cell cytoskeleton: PL124 and PL188 showed a similar trend, reaching the peak at 1.5h (Figure 5C); in the cell nucleus: the intake of PL124 peaked at 2 h and PL188 at 1.5 h, but the intake of PL124 was higher than that of PL188 (Figure 5D).
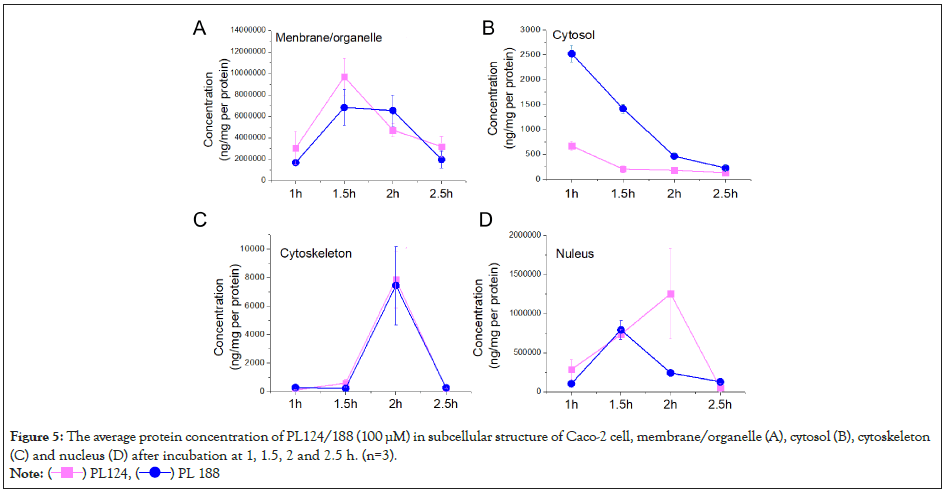
Figure 5: The average protein concentration of PL124/188 (100 μM) in subcellular structure of Caco-2 cell, membrane/organelle (A), cytosol (B), cytoskeleton
(C) and nucleus (D) after incubation at 1, 1.5, 2 and 2.5 h. (n=3).
Note: ( ) PL124, (
) PL124, ( ) PL 188
) PL 188
The uptake and efflux of Caco-2 in intestinal cells of PL124 and PL188 were different
Active transport is the transport mode of most drugs, which is characterized by a relatively small concentration difference between the two sides, which can facilitate the control of drug delivery and intestinal absorption at a lower concentration, contributing to the efficient function of drugs [27]. The results showed that PL124 and PL188 were actively transported and absorbed by Caco-2 cells. Therefore, both have the potential to be highly effective oral drugs in this respect. Since most of the active transport modes enter, generally separated the endocytosis and pinocytosis pathways, and are controlled by different inhibitors, such as colchicine (pinocytosis inhibitors), chlorpromazine (clathrin mediated endocytosis inhibitors), buxanthine (fossa mediated endocytosis inhibitors), and quercetin (independent of clathrin and fossa mediated endocytosis inhibitors)[28-31], a single switch is turned off. The reduced amount of the drug entering the cell indicates that it is dependent on the receptor of the pathway to enter the cell. Further, we found that PL124 and PL188 enter cells differently: The accumulation of Poloxam 188 in cells was significantly reduced under the inhibition of colchicine, gorse isoflavone and quercetin. Therefore, it can be concluded that Poloxam 188 enters cells through three endocytosis pathways, namely pinocytosis pathway, fosselin-mediated endocytosis pathway, and the endocytosis pathway independent of mestin and fosselin-mediated endocytosis pathway. More surprisingly, PL124 intake was significantly increased after chlorpromazine and colchicine were added, suggesting that PL124 may be ingestion by other means independent of endocytosis and pinocytosis, which requires further experiments to verify.
Pgp protein is widely found in cell membrane, and is the main switch for drugs to be pumped out of cells after entering cells. Generally, highly expressed Pgp exists in a variety of tumor drug resistant cells, which is the main cause of tumor drug resistance [32]. In this result, we found that PL124 and PL188 have different effects on the secretion of P-gp protein substrate Rho123 on the cell membrane of Caco-2, and PL188 has a stronger effect than PL124, which indicates that PL188 may have a greater interaction with P-gp on Caco-2. This suggests a tendency to pump Caco-2 cells more strongly, which is characteristic of the use of PL124 and PL188 against drug resistant diseases such as tumors, especially intestinal tumors.
The accumulation of suborganelles of Caco-2 in intestinal cells of PL124 and PL188 were different
The level of suborganelle accumulation membrane/organelle, cytosol, cytoskeleton and nucleus) may play an important role in affecting the play of some functional enzymes, for example, nucleation may be involved in regulating the function of some functional proteins. This is especially important in the treatment of intestinal diseases [19]. There are many proteins on the cell membrane, such as P-gp protein, which control many different functions [32]. The cytoskeleton also provides many important clues for the maintenance of the shape and normal growth of many cells [33], while there are many important enzymes and nutrients in the cytoplasm, which play an important role in important functions [34]. PL124 rapidly and more reaches the cell membrane than PL124, PL188 rapidly reaches the cytoplasm and nucleus, and more in cytoplasm than PL124, both stays in the skeleton.
These results indicate that different molecular weights of PL124 and PL188 accumulate in different subcells, which may affect the corresponding function. The specific results need to be verified by further experiments.
The uptake in Caco-2 cells are different: Both PL188 and 124 entered Caco-2 cells through active transport, but PL188 uptake into Caco-2 cells relies on pinocytosis pathway, fosselin-mediated endocytosis pathway, and the endocytosis pathway independent of mestin and fosselin-mediated endocytosis. While PL124 uptake by independent of clathrin- and fossa - mediated endocytosis inhibitors pathway; The effects of PL188 on P-gp protein substrate were different: PL188 was stronger than PL124; There are also differences in subcellular accumulations: PL124 rapidly and more reaches the cell membrane than PL124, PL188 rapidly reaches the cytoplasm and nucleus, and more in cytoplasm than PL124, both stays in the skeleton (Figure 6). This work provides data basis for our subsequent application of PL188 and PL124 as oral preparations.
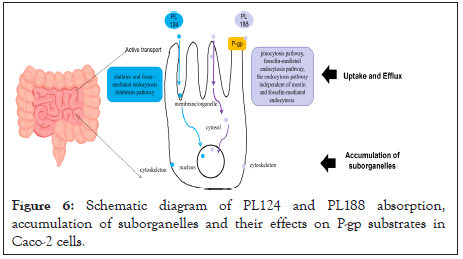
Figure 6: Schematic diagram of PL124 and PL188 absorption, accumulation of suborganelles and their effects on P-gp substrates in Caco-2 cells.
The data underlying this article are available in the article and in its online supplementary material.
The authors declare no conflict of interests.
Xiaoying Lin: Investigation, Methodology, Data curation, Validation, Formal analysis.
Xinxin Zhou: Investigation.
Feng Hao: Cell experiment site.
Lele Li: Investigation, Software, Visualization, Writing - original draft.
Yue Cui: Writing - original draft.
Yanfei Zhang: Investigation.
Heyun Zhu: Project administration, Supervision, Writing - review & editing.
Bo Feng: Conceptualization, Funding acquisition, Supervision.
This study was supported by the National Natural Science Foundation of China (81773692) and the Natural Science Foundation Project from the Department of Science and Technology of Jilin Province (20180101187JC). Jilin Province Health and Healthy Youth Science and Technology Key Training Program project (2020Q030), Science and Technology Research Project of Jilin Province Education Department(JJKH20210496KJ).
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar].
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Lin X, Zhou X, Hao F, Li L, Cui Y, Zhang Y, Guan J, Zhu H, Feng B (2023) Molecular-Mediated Difference Action of Poloxham124/188: Absorption, Efflux and Suborganelle Accumulation in Caco-2 Cells. J Drug Metab Toxicol. 14:285
Received: 21-Feb-2023, Manuscript No. JDMT-23-21874; Editor assigned: 23-Feb-2023, Pre QC No. JDMT-23-21874(PQ); Reviewed: 10-Mar-2023, QC No. JDMT-23-21874; Revised: 17-Mar-2023, Manuscript No. JDMT-23-21874(R); Published: 24-Mar-2023 , DOI: 10.35248/2157-7609.23.14.285
Copyright: © 2023, Lin X et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.