
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2024)Volume 7, Issue 1
Gastric cancer is globally recognized as a significant malignancy and a leading cause of cancer mortality. Ferroptosis, as a new form of regulated cell death, is attracting increasing attention in worldwide. As knows that, MicroRNA-365 (MiR-365) has been implicated in the carcinogenesis of gastric cancer, but its role in ferroptosis remains elusive. The aim of our study is to clarify how miR-365 regulate ferroptosis in gastric cancer cells.
Erastin is regarded as a ferroptosis inducer, our study found that with over-expression of miR-365 mimics also could enhance erastin-induced ferroptosis in gastric cancer cells. Additionally, miR-365 overexpression further upregulated the levels of MDA, Fe2+ and ROS in gastric cancer cells exposed to erastin, while antioxidant GSH levels were further downregulated, suggestive of enhanced lipid oxidation in gastric cancer cells upon erastin treatment through miR- 365 overexpression.
Notably, Nuclear factor erythroid 2-Related Factor 2 (NRF2) participated in the ferroptosis of cancer cells, as its upregulation was observed in gastric cancer cells after erastin exposure. qRT-PCR and Western blot analysis has shown that after exposed with erastin, the transcription and translation levels of Nrf2 in SGC-7901 and MGC-803 cells was increased. Overexpression of miR-365 in these cells attenuated the induction of Nrf2 by anti-aging drugs. The luciferase reporter assays data providing the evidence that miR-365 could directly target Nrf2.
In conclusion, our study demonstrates that microRNA-365 could directly targets Nrf2. And its expression enhances ferroptosis induced by erastin in gastric cancer cells. This may provide a new target for therapy of gastric cancer regarding ferroptosis.
Nrf2; Ferroptosis; MicroRNA, MiR-365; Gastric cancer
Gastric cancer is ranked as the fifth most prevalent malignancy worldwide and the third leading cause of cancer related mortality, with over one million new gastric cancer diagnoses and over 750,000 gastric cancer deaths in 2020 alone [1,2]. Gastric Adenocarcinoma (GAC) the most common histological subtype of gastric cancer, is responsible for the high mortality rate, which is the result of a combination of multiple factors. Conventional anti-tumor drugs currently available have limited utility [3].
Ferroptosis, which is defined as a new form of cell death, is characterized by distinct morphological features, including smaller mitochondrial volume, increased density of bilayer membranes and loss or reduction of mitochondrial cristae, while the cell membrane remains intact. The nucleus maintains its normal size and chromatin does not condense [4]. Small molecule compounds, such as erastin or RAS-Selective Lethal 3 (RSL3), function by inhibiting the uptake of cysteine or targeting the phosphoperoxidase Glutathione Peroxidase 4 (GPX4), leading to the breakdown of redox homeostasis and ferroptosis [5,6]. These ferroptosis inducers play an anti-tumor role in many types of malignant tumors [7-10]. However, cancer cells have the ability to activate or reshape stress pathways to prevent cell death, thereby limiting the activity of ferroptosis inducers [11]. Many downstream target genes of Nrf2 are involved in maintaining and preventing cellular redox balance and it is considered a crucial regulator of antioxidant response.
The sensitivity of ferroptosis is directly linked to Nrf2 is a key transcription factor for antioxidant response, as increasing Nrf2 expression can prevent ferroptosis, inhibiting Nrf2 can increase the sensitivity of cancer cells to pro-ferroptotic drugs [12,13].
MicroRNA (MiRNA) is a small nucleic acid that plays an important regulatory role in controlling gene expression [14]. MicroRNAs participate in the development of cancer, such as miR-34a and miR-96-5p [15,16] and some of them are found to be related to ferroptosis in recent studies [17,18]. In recent studies, miR-365 has received attention as a regulatory factor for Nrf2 [19,20]. It has been reported an increased expression of microRNA-365 may inhibit the malignant development of gastric cancer [21]. MiR-365 together with metformin could also promote apoptosis of gastric cancer cells [22]. However, it is still unclear whether the miR-365- Nrf2 axis will affect ferroptosis of cancer cells.
This study is focused on the role of miR-365 in ferroptosis in two gastric cancer cell lines. Our findings indicate that miR-365 enhanced erastin-induced ferroptosis in gastric cancer cells by targeting Nrf2.
Cell culture, transfection and treatment
Human gastric mucosal epithelial cell GES-1, human gastric adenocarcinoma cell SGC-7901 and human gastric cancer cell MGC-803 were obtained from the Chinese Academy of Sciences (Shanghai, China) and cultured in an essential medium supplemented with 10% bovine serum at 37°C with 5% CO2. SGC-7901 and MGC-803 cells were first transfected using OV- Pre-miR-365, anti-miR-365 or transfected with Negative Control (NC) vectors, then, the cells were screened with 150 or 200 μg/ml G418. Cells were treated with erastin, Ferrostatin-1, ZVAD-FMK or necrosulfonamide for 24 hours.
Tissue RNA isolation
Followed by the manufacturer's protocol of Trizol reagent (Invitrogen, USA), total Ribonucleic acid (RNA) were isolated from tissues and cells. The concentration and quality of the isolated RNA were assessed by using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific).
Quantitative real-time PCR
Quantitative Real Time Polymerase Chain Reaction (qRT-PCR) of miRNA was performed using the miRNA Universal SYBR qPCR master mix (Vazyme, China). The qRT-PCR reaction was performed in the ABI7500 system (Applied Biosystems, CA, USA). The relative quantification values for microRNA were calculated by the 2-ΔΔCT method using U6 as an endogenous control. Table 1 below contains the primers used in this study.
| Primer name | Sequences (from 5’-3’) |
|---|---|
| miRNA-365-F | CGTAATGCCCCTAAAAAT |
| miRNA-365-R | GTGCAGGGTCCGAGGT |
| U6-F | CTCGCTTCGGCAGCACA |
| U6-R | AACGCTTCACGAATTTGCGT |
Table 1: Quantitative RT-PCR primers.
Cell viability assay
Cell Counting Kit-8 (CCK-8, KeyGEN Biotech) were used to assess cell viability. Briefly, 3000 cells in each well were seeded in 96-well plate. After incubating 24 h, treated cells with drug for another 48 h. Then, 10 μl CCK-8 was added to each well, then incubated for 1 h at 37°C. The optical density at 450 nm was measured and analyzed by using a microplate reader (MD SpectraMax iD3).
Clone formation assay
After cell transfection and drug incubation, 300 cells were seeded in 24-well plate. After 14 days, cells were fixed with 4% paraformaldehyde and stained with Wright-Giemsa (NJJCBIO, China). Colonies were imaged under a microscope number of colonies was counted.
Malondialdehyde, Fe2+ and glutathione assay
Cell protein were isolated and measured with a Bicinchoninic Acid (BCA) kit (Beyotime, China). Levels of Malondialdehyde (MDA), Fe2+ and Glutathione (GSH) were measured using corresponding commercial kits (NJJCBIO, China) in accordance with the manufacture’s protocols, respectively.
Luciferase reporter assay
In order to analyze miR-365 targets, both Wild Type (WT) 3′- UTR and Mutant (MUT) 3′-UTR of Nrf2 containing the miR- 365 binding site was cloned into the pMIR‐GLO dual luciferase miRNA target vector. For the luciferase assay, Lipofectamine 2000 was used to co-transfect 293 T cells with the miR‐365/miR-NC and dual luciferase vectors. After 48 hours, luciferase activity was measured using the Dual Luciferase Reporter Gene Assay Kit (Beyotime, China), firefly luciferase activity was normalized to Renilla luciferase activity.
Reactive oxygen species measurements
Reactive Oxygen Species (ROS) level were measured using ROS Assay Kit according to the kit's protocol (Beyotime, China). In short, after collected cells, then incubated the cells with Dichloro- Dihydro-Fluorescein Diacetate (DCFH-DA) at 37°C for 20 min. After washed by Phosphate Buffered Saline (PBS), the ROS level was analyzed via a microplate reader (MD SpectraMax iD3).
Western blot analysis
After washed by PBS, cells then lysed with Radio- Immunoprecipitation Assay (RIPA) lysis buffer supplemented with 1% phenylmethylsulfonyl flouride (Solarbio, China). After removing debris by centrifuging, protein concentrations in the supernatant were qualified with a BCA kit (Solarbio, China). 20 μg protein of each sample were loaded to SDS-PAGE gel. Polyvinylidene Difluoride (PVDF) membranes (Millipore) were used to transfer protein and blocked with 5% nonfat milk for 1 hr. The primary antibody incubating at 4°C overnight for the binding. Then, secondary antibodies were incubated for 1h at RT.
Statistical analysis
The data was presented as the mean ± standard deviation. Statistical analyses between two samples were performed using student's t-test. Comparison of data among multiple groups was conducted using one-way analysis of variance followed by Tukey's test. A p-value less than 0.05 was considered statistically significant. GraphPad Prism version 8 was used for data analysis.
MiR-365 is downregulated in different gastric cancer cell lines and tissues
lines and tissues The relative quantitative changes in miR-365 expression in cancer samples were measured using qPCR. Compared with normal gastric mucosa tissue, miR-365 levels in all tumors decreased (Figure 1a). In addition, SGC-7901 and MGC-803 cells showed a decrease in miR-365 compared to normal cells (Figure 1b). These results suggest that changes in miR-365 expression may be related to the progression of gastric cancer.
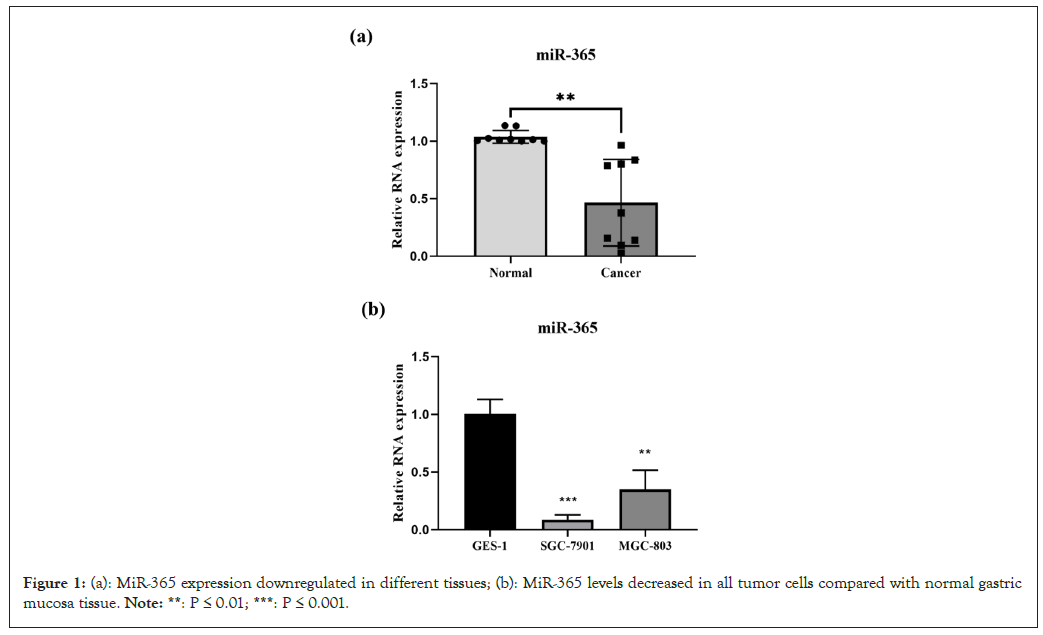
Figure 1: (a): MiR-365 expression downregulated in different tissues; (b): MiR-365 levels decreased in all tumor cells compared with normal gastric mucosa tissue. Note: **: P ≤ 0.01; ***: P ≤ 0.001.
MiR-365 enhances erastin-induced ferroptosis in gastric cancer cells
Firstly, we checked the sensitivity of cells to ferroptosis. The vitality of SGC-7901 and MGC-803 which was treated with erastin alone or with ferroptosis inhibitor (Ferrostatin-1), apoptosis inhibitor (ZVAD-FMK) and necroptosis inhibitor (necrosulfonamide) were measured by CCK-8. As shown in Figure 2a, ferroptosis inhibitor reversed cell death induced by erastin, whereas the apoptosis and necroptosis inhibitors did not show the same effect, which proved that SGC-7901 and MGC-803 cancer cells exhibited susceptibility to the ferroptosis inducer.
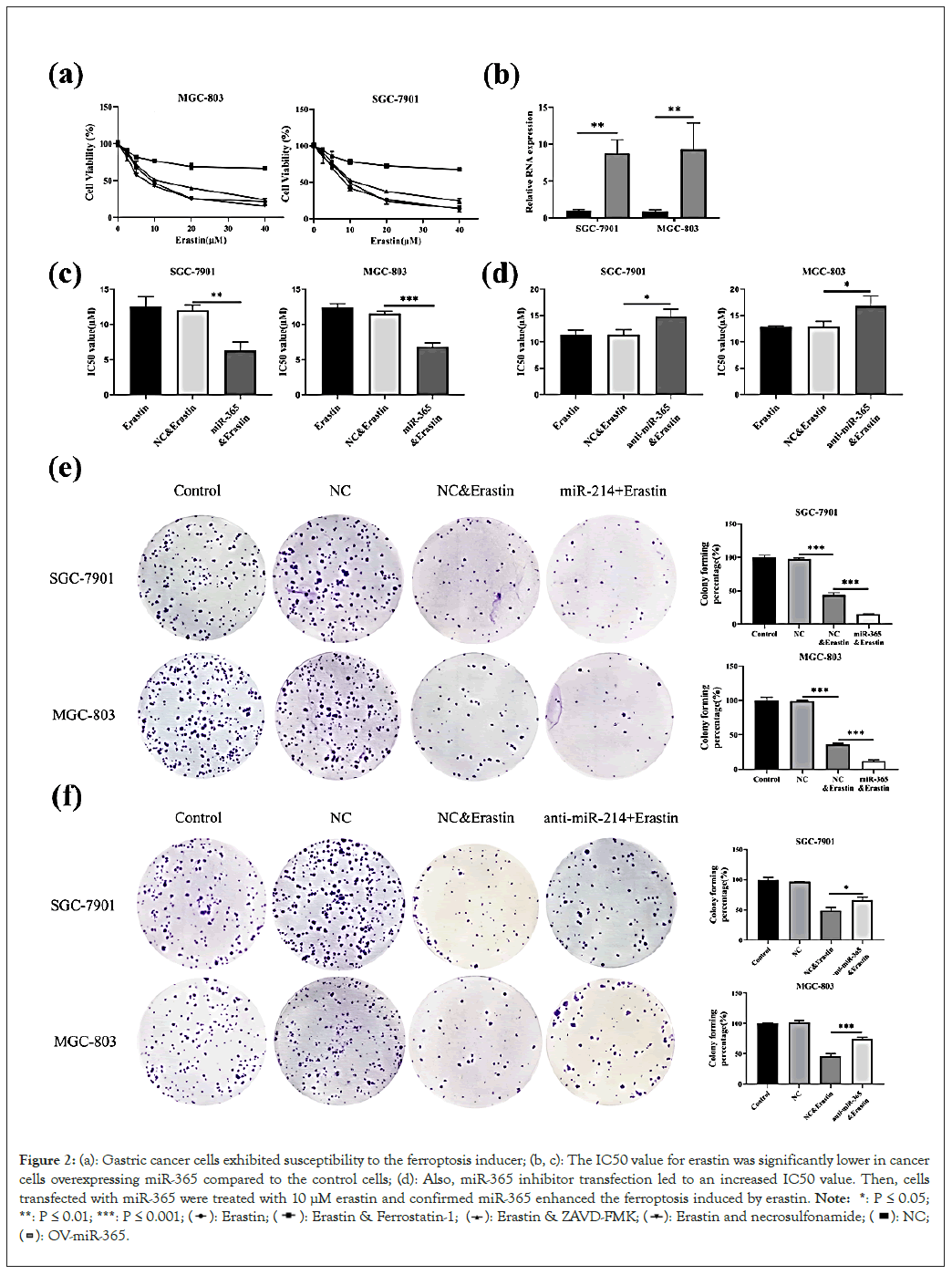
Figure 2: (a): Gastric cancer cells exhibited susceptibility to the ferroptosis inducer; (b, c): The IC50 value for erastin was significantly lower in cancer cells overexpressing miR-365 compared to the control cells; (d): Also, miR-365 inhibitor transfection led to an increased IC50 value. Then, cells transfected with miR-365 were treated with 10 µM erastin and confirmed miR-365 enhanced the ferroptosis induced by erastin. Note: *: P ≤ 0.05; **: P ≤ 0.01; ***: P ≤ 0.001; 
Next, SGC-7901 and MGC-803 cells were transfected with miR- 365 mimics. Compared with control cells, half maximal Inhibitory Concentration (IC50) value for erastin was significantly lower in cancer cells overexpressing miR-365 compared to the control cells. (Figures 2b and 2c). At the same time, miR-365 inhibitor transfection led to an increased IC50 value (Figure 2d). Then, cells transfected with miR-365 mimics were seeded and cell colonies were treated with 10 μM erastin for 2 weeks. These findings confirmed that miR-365 enhanced the ferroptosis induced by erastin (Figures 2e and 2f).
MiR-365 augments erastin-induced lipid oxidation in gastric cancer cells in vitro
SGC-7901 and MGC-803 cells were transfected with miR365 mimics or NC and treated with 10 μM erastin for 24 hours. MDA, Fe2+, ROS and GSH levels were measured using commercially available kits. We found that miR-365 mimics further up-regulated the MDA, Fe2+, ROS levels in SGC-7901 and MGC-803 cells after erastin exposure (Figures 3a-3c). On the contrary, antioxidative GSH levels were further down-regulated (Figure 3d). These results indicate that lipid oxidation in gastric cancer cells induced by erastin could be enhanced by overexpression of miR-365.
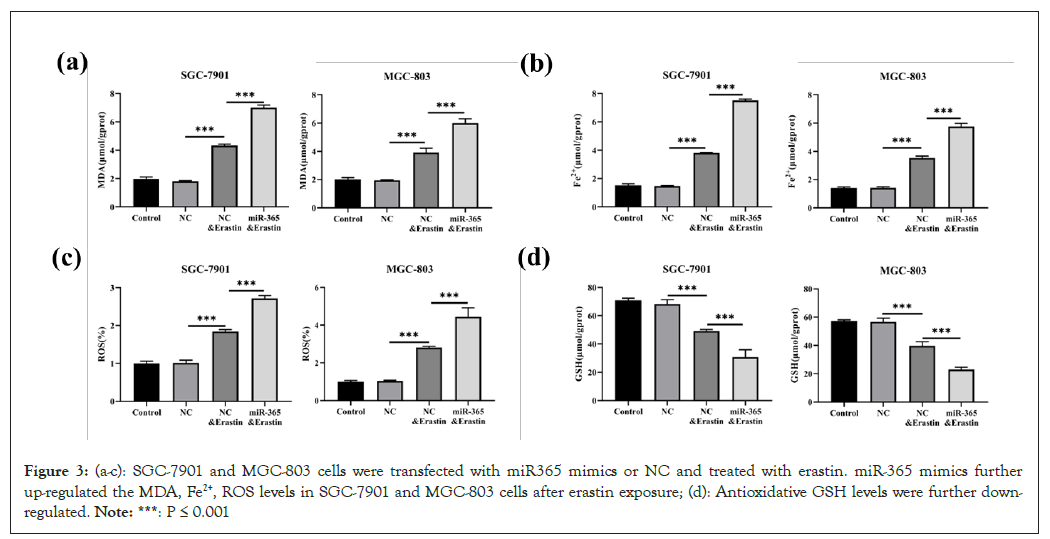
Figure 3: (a-c): SGC-7901 and MGC-803 cells were transfected with miR365 mimics or NC and treated with erastin. miR-365 mimics further up-regulated the MDA, Fe2+, ROS levels in SGC-7901 and MGC-803 cells after erastin exposure; (d): Antioxidative GSH levels were further down-regulated. Note: ***: P ≤ 0.001.
MiR-365 directly targets Nrf2
Nrf2 took part in the ferroptosis of cancer cells [23]. Besides, the expression of Nrf2 was observed in gastric cancer cells after erastin exposures. qRT-PCR and Western blot analysis results demonstrated that erastin increased the transcription and translation levels of Nrf2 in SGC-7901 and MGC-803 cells (Figures 4a and 4b). When miR-365 is overexpressed in these cancer cells, the prevention of the increase in Nrf2 induced by anti-aging drugs is observed. As evidenced by the luciferase analysis data, compared to NC simulation, miR-365 simulation could reduce the activity of luciferase to the wild type Nrf2 3’-UTR. But it did not show any effect on the activity of luciferase to the mutation type Nrf2 3'-UTR (Figures 4c and 4d). These findings could provide evidence that miR-365 could directly target Nrf2.
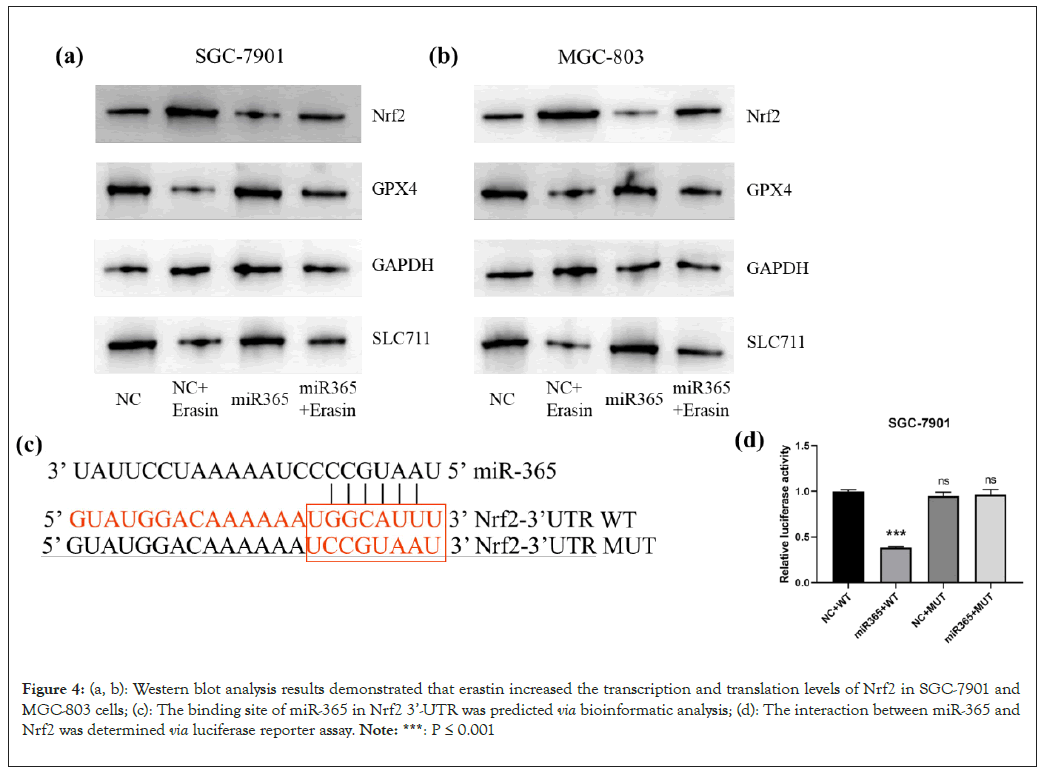
Figure 4: (a, b): Western blot analysis results demonstrated that erastin increased the transcription and translation levels of Nrf2 in SGC-7901 and MGC-803 cells; (c): The binding site of miR-365 in Nrf2 3’-UTR was predicted via bioinformatic analysis; (d): The interaction between miR-365 and Nrf2 was determined via luciferase reporter assay. Note: ***: P ≤ 0.001.
Based on the latest studies, we demonstrated that miR-365 could make SGC-7901 and MGC-803 cells become more sensitive to erastin, which is a classic ferroptosis inducer, by inhibiting the expression of Nrf2.
Erastin was firstly ascertained as an inducer of cell death in 2003 [24]. As ferroptosis was named by Dixon in 2012 [4], Hao S and others [25-27] have shown that the induction of erastin can result in the death of gastric cancer cells, which is suggesting that gastric cancer cells are sensitive to the inducers of ferroptosis. Erastin suppress the synthesis of cysteine-dependent GSH, hence caused the promotion of the toxic ROS accumulation and lipid oxidation products, MDA [28,29]. By assessing the changes in these molecules associated with lipid oxidation, erastin induced the oxidation of lipids in gastric cancer cells was observed.
An interesting observation was found during this study. It was revealed that miR-365 significantly enhances erastin-induced cell death. However, it is still unclear about how miR-365 affecting in the pathogenesis of oxidative stress-related retinopathy. Juan Wang, et al. believe that miR-365 is associated with muller cell gliosis through oxidative stress aggravation [30]. Ming Gao, et al. found miR-365 served as an intermediate between MT1DP and Nrf2, also at the translation level, it plays a vital role in enhancing Cd-induced oxidative stress by reducing Nrf2 concentrations [20]. Jia Lin Mo, et al. found that upregulation of miR-365 intensifies ischemic neuronal damage through increased oxidative stress by targeting Oxidation Resistance Protein 1 (OXR1). Conversely, silencing miR-365 provided protection against ischemic neuronal injury by activating OXR1-mediated antioxidant signaling [31]. It is revealed that miR-365 not only further enhances the erastin- induced reduction of GSH, but also accumulates the ROS and MDA in gastric cancer cells. Moreover, our findings elucidate the function of miR-365 in stimulating ferroptosis in gastric cancer cells.
It is complicated about the function of Nrf2 in regulating ferroptosis in cancer cells. Nrf2 plays an important role in suppressing cell damage, oxidative stress and inflammation, which can potentially inhibit tumor initiation. However, strong activation of Nrf2 through genetic alterations or other mechanisms has been observed in various types of cancers. This enhanced Nrf2 activation enables cancer cells to adapt to the microenvironment of hostile, promoting metabolic modifications that support rapid cancer cell proliferation, making tumor grow and invasion [32]. Besides, increasing their sensitivity towards chemotherapeutic agents in gastric cancer cells was observed upon the inhibition of Nrf2 [33,34]. Our experimental data has proved that miR-365 downregulated the transcription of Nrf2 and reduced its protein expression in SGC-7901 and MGC-803 cells which was exposed to erastin. Based on our deduction, the observation that miR- 365 enhanced cell death triggered by erastin, suggesting that the downregulation of Nrf2 played a key role in miR-365-induced ferroptosis. Through the deletion of the miR-365 binding site in Nrf2 3’-UTR, we were able to confirm the direct targeting of Nrf2 by miR-365. In this article, it was first time to be unveiled that miR-365 sensitizes gastric cancer cells to ferroptosis inducers by destabilizing Nrf2.
The microenvironment within tumors exhibits a significantly higher level of complexity compared to that of cultured cells. To comprehensively investigate the role of the miR-365-Nrf2 axis in cellular ferroptosis, future studies should include in vivo experiments and the use of additional ferroptosis inducers.
This study aimed at whether miR-365 could regulate ferroptosis of gastric cancer cells. Results showed that miR-365 downregulated in cell lines and tissues. Moreover, expression of miR-365 mimics enhanced the erastin-induced ferroptosis in gastric cancer cells. Additionally, miR-365 mimics further upregulated the MDA, Fe2+, ROS levels in SGC-7901 and MGC-803 cells after erastin exposure and antioxidative GSH levels were further downregulated, which indicate that lipid oxidation in gastric cancer cells induced by erastin could be enhanced by overexpressing miR-365. Nrf2 took part in the ferroptosis of cancer cells, it was observed in gastric cancer cells after erastin exposures. qRT-PCR and Western blot results showed that erastin increased the transcription and translation levels of Nrf2 in gastric cancer cells. The luciferase data confirmed that miR-365 directly targets Nrf2. To compare with NC simulation, miR-365 simulation could reduce the activity of luciferase to Nrf2 3’-UTR wild type but no effect on the mutation type, which could provide evidence that miR-365 could target Nrf2.
The research data used to support the findings of this study are included within the article.
The authors declare that there is no conflict of interest regarding the publication of this paper.
Hui, C, Ming, D, Kai, Z have made a substantial contribution to the study.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[CrosssRef] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Cao H, Ding M, Zhao K (2024) MicroRNA-365 Targets Nrf2 to Enhance Erastin-Induced Ferroptosis in Gastric Cancer Cells. J Clin Chem Lab Med. 7:284.
Received: 25-Oct-2023, Manuscript No. JCCLM-23-27782; Editor assigned: 27-Oct-2023, Pre QC No. JCCLM-23-27782 (PQ); Reviewed: 10-Nov-2023, QC No. JCCLM-23-27782; Revised: 17-Nov-2023, Manuscript No. JCCLM-23-27782 (R); Published: 24-Jan-2024 , DOI: 10.35248/2736-6588.24.7.284
Copyright: © 2024 Cao H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.