Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Review Article - (2015) Volume 6, Issue 1
Depleting reserves and souring prices of petroleum and oil have daunting effect on the economy of many developed and developing nations and forced researchers, government and federal agencies to opt for development of alternate fuels. In recent years, there is renewed interest in the field of algal biofuel production owing to its ability to grow in non-agriculturalable waste land and municipal wastewater/agricultural runoff water. Previous decade was attributed in developing new open culture/ reactor design for enhanced microalgal biomass production, efficient harvesting and pretreatment systems for commercial algal biofuel production. The true potential of algal biofuel spurs debate between algae experts and external observer over economics and environmental sustainability. Currently, no commercial algal energy generation system, which can surpass crop based energy content, is available. In present review, efficient culturing, operational strategies, reactor design and technological advancement in processing of algal fuel have been reviewed in graphical abstract
Keywords: Biofuel; Algal fuel; Biorefinery; Microalgae
Depleting fossil fuel reserves, global warming and increased global demand for energy pushed the world to a stage where finding a new renewable green alternative fuel is inevitable. Presently the United States and China are the largest worldwide oil consumers (>10 million barrel per day) followed by Japan (4.7) and India (3) (US Energy Information Administration). It is estimated that by the year 2020, there will be 60 fold increases in the demand for oil worldwide. The fossil oil discovery has declined steadily (from 55 billion barrel per year to 10 billion barrel) since 1960’s and extractions from unconventional natural sources (deep wells, oil shale and tar sands) are difficult and much more expensive [1].
The noteworthy progress has been made in improving technologies for biological conversion/biofuel production though capital costs for biofuel facilities are relatively high. Although commercial momentum is building, but this emerging industry is still facing many challenges. However, issues like improvement in understanding of the fundamental principles of biofuel efficiency, its scale up technology development and how it will change the automobile sector must be addressed.
The photosynthetic organisms are the most energy efficient systems present in nature and they can form valuable renewal raw material using photoautotrophic mode. Among these photosynthetic organisms algae form a fascinating group of ubiquitous organisms that has high biomass yield, high lipid or/and starch content, that can easily grow on effluents like wastewater and carbon dioxide on waste land. Algae based fuels are environmental friendly because they convert the CO2 produced from burning of fuel again to algal biomass. There have been lot of controversies about carbon neutrality of biofuels because calculation of the carbon neutrality is a complex and imprecise method based highly on the assumptions. However, virtually all carbon neutral fuels actually require burning of fossil fuel in pre or post production stages (process equipments, harvesting, transport of feed stock to the biorefinery, converting the feedstock into ethanol/biodiesel, and further transporting the biofuel to a petroleum refinery or service station). But the overall CO2emmision/ production is less as compared to other fuels [2-4] .
Due to its chemical characteristics, algal fuel resembles gasoline and mix freely with fossil fuels and they could be used in existing automobile infrastructure. Biomass productivity of the algae is ten times as compared to crops and it could be cultivated in the desert. These characteristics make them attractive raw material for bioenergy production (Figure 1). Earlier the oil productivity was predicted to be more than 100 tons/ha per year (~10 Kg/m2 per year), although till recent times only 40-60 tons/ha per year (~4-6 Kg/100 Kg/m2 per year) was achieved commercially and 30 tons of biodiesel/ha per year (~3 kg/ m2 per year) was produced in subtropical or tropical regions [5].
Currently few companies are developing technologies for sustained algal biomass production for producing biofuels but raw data and technological facilities used in the process is not accessible, may be due to patenting issues. Nevertheless the large investment from venture capital, Government grants, collaboration between US large companies and academic institutions in last decade in algae biomass production fueled the idea that algae certainly hold some promise as future fuel [6,7]. In present manuscript we will discuss technological advancement achieved in previous years and the potential of algae as future fuel along with the challenges ahead.
Why algae as a renewable resource?
The agriculture practice is changing drastically in many countries e.g. in Indonesia tropical forest were destructed to grow fuel crops like palm oil and hence posed adverse ecological impact. These crops demand enormous agricultural land, water and fertilizers and yield less than 500-5,000 L of biodiesel per hectare [8]. In comparison algae can be grown on effluents and agricultural and municipal runoff water often can be used in conjugation with wastewater treatment and hold promise as a suitable raw material.
Algae contain carbohydrates and lipids as the main components of biomass. Algae has >50% of its mass in the form of carbohydrate, hence algal dry biomass is an important source to produce fermentable sugar from cellulose and hemicellulose which can be easily converted into bioethanol [9].
Algae has a unique cell wall composed of mainly polysaccharides and no lignin as compared to other lignocellulosic waste which pose considerable restriction to get fermentable sugars from agricultural waste. A major breakthrough came when Deng and Coleman reported that genetically engineered green algae can be exploited for ethanol production from sunlight e.g. NREL (National Renewable Energy Laboratory) uses Chlorophyceae (green algae) and diatoms [10]. Green algae grow rapidly in nitrogen at 30°C, while diatoms require silicon for growth. Algenol Ltd. attracted significant funding when they transformed cyanobacteria to cyanobacterila strain using recombinant DNA technology which got the ability to transform the photosynthetically synthesized sugar into ethanol and also excrete it into the growth medium [11].
Algal oil usually consist of shorter chains and highly saturated lipids (C14:0, C16:0 and C16:1) which are generally favorable for fuel production. Lipid content of algae varies from 2-60% of dry weight (Chlorella sp. 28-32%, Cylindrotheca sp. 16-37%, Phaeodactylum tricornutum 20-30%). In diatoms Hantzschia and Nitzschia produce about 66% (dry weight) and 28-50% (dry weight) of oil (Table 1). Some algae like Schizochytrium sp. And Botryococcus braunii can accumulate ~75% of lipid and it can be converted to Straight Vegetable Oil (SVO) or biodiesel [12]. Microalgal oil yield per acre is about 10-25 times (5.87-13.69 L/m2) as compared to rich plant oil source like palm oil (0.5949 L/m2). The increased lipid content of algae can be achieved either by strain selection (having high growth rate and oil content) in open culture and closed bioreactors or by manipulating conditions [13]. In algae environmental stress like nutrient deficient environment often results in increase lipid accumulation >60% [14-16], however, these algal oils are unsaturated and hence cannot be used directly in sensitive engines.
| Name of Alga | Oil (% Dry weight) | Commercial culture system |
|---|---|---|
| Ankistrodesmus | 28-40 | Tank |
| Botryococcus braunii | 29-75 | Open raceway pond |
| Chlorella protothecoides | 15-55 | Central Pivot Ponds, Raceways, Tubular Photobioreactors |
| Chlorella sp. | 29 | Central Pivot Ponds, Raceways, Tubular Photobioreactors |
| Crypthecodinium cohnii | 20 | Fermenter (heterotrophic) |
| Cyclotella | 42 | - |
| Cylindrotheca sp. | 16-37 | - |
| Dunaliella tertiolecta | 36-42 | Extensive ponds |
| Haematococcus | 20-35 | Open paddle-wheel pond , Tubular Photobioreactors |
| Hantzschia | 66 | - |
| Isochrysis sp. | 25-33 | - |
| Monallanthus salina | >20 | - |
| Nannochloris | 31 (6-63) | - |
| Nannochloropsis | 46 (31-68) | Raceways |
| Neochloris oleoabundans | 35-54 | - |
| Neochloris oleoabundans | 35-54 | - |
| Nitzschia | 28-50 | - |
| Phaeodactylum tricornutum | 31 | Vertical bubble column and airlift photobioreactors |
| Pleurochrysis carterae | Raceways | |
| Scenedesmus | 45 | - |
| Schizochytrium | 50-77 | - |
| Spirulina | 4-11 | Raceways |
| Stichococcus | 33 (9-59) | - |
| Tetraselmis suecica | 15-32 | Big bag culture |
| Thalassiosira pseudonana | 21-31 | - |
Table 1: Representing Alga against the amount of Oil obtained as the% dry weight along with the Commercial Culture system that was used to obtain biofuel.
Botryococcus braunii is well known cosmopolitan planktonic fresh water microalga, because of its ability to produce hydrocarbon in the form of triterpenes, which gets accumulated on the outside of the cell [17,18]. Generally hydrocarbon consists of 20-60% of algal dry mass and maximum production up to 86% has also been reported [19,20]. It produces three major types of hydrocarbons: botryococcenes, alkadienes and alkatrienes (C23-C33 alkenes). The Botryococcus hydrocarbons are non-edible and are very different in chemical constituents of vegetable oil. These hydrocarbons cannot be directly trans-esterified because these triterpenes do not possess the free oxygen atom which is required for trans-esterification reaction but they can produce octane (gasoline/petrol) by hydro-cracking in refinery. On hydro-cracking, Botryococcenes hydrocarbon results into a fuel with high octane value as compared to alkadienes and alkatrienes [21]. Many research groups are studying B. braunii growth profile in high salt concentration, Navailability and phosphate, light intensity and pH 8 [22]. The major drawback is that it show fastidicious growth and extraction is difficult because of thick cell wall, which make them unsuitable for large scale biofuel production. Hydrocracking of these hydrocarbons produce various fuel types like diesel, gasoline and kerosene.
Algae can also be used as a source for hydrogen production which can be achieved by three methods: biophotolysis of water, gasification and Steam reformation (SMR) of methane. They form one of the most promising future fuels because they produce only water as an exhaust product and not NOx gases. Only algae synthesize hydrogen directly from sunlight and water in the complete anaerobic conditions [23], while other microbes like non-sulphurbacteria and green sulphur bacteria use solar energy to extract hydrogen gas from wide variety of substrates [24].
In Scenedesmus and Chlorella vulgaris, hydrogenase-dependent hydrogen production was observed when alga was grown in anaerobic conditions [25,26]. Kansai Electric Power Co. Ltd. (Nankoh, Osaka, Japan) established a pilot plant for hydrogen generation by clubbing green algae and photosynthetic bacteria. Arthrospira species are reported to possess highest hydrogen producing activity. Nitrogenase mediated hydrogen production under aerobic conditions was proposed by Asada and Kawamura in Anabaena sp. [27]. Chlamydomonas reinhardtii produces hydrogen in sulfur deprived medium in biohydrogen reactor [28,29]. Hence for algal hydrogen gas production only closed culture system can be employed. Currently only a fraction of H2 gas production using algae has been achieved of its theoretical maximum (i.e. 20 g H2/m2/day) value, making process commercially unviable. In the year of 2013, Grow Energy Co. developed Hydral bioreactors for the commercial production of hydrogen using genetically modified algae [30]. In the same year, in Falkenhagen (Germany), the first commercial 2 megawatt power to gas installation was established which generate 360 m3 of hydrogen per hour [31].
Culturing techniques of algae
Microalgae are phototrophic organisms that can increase their biomass using light, carbon dioxide, water, and inorganic salts [32]. The optimal growth temperature varies from 15-30°C and agitation is required to prevent algal biomass settlement to the bottom [33]. Respiration during the night hours causes some part of the biomass loss produced during day time [34]. The microalgae usually grow on attached surfaces but it prevent the gas and light penetrance to lower level when it outgrow hence causing the death of underlying layer, thus for mass production usually suspension cultures are preferred. The predicted yield of algal biomass from pilot scale to fermenter scale is mostly erroneously calculated because in high density culture, underlying algae are unable to capture all incoming light to convert it into biomass, hence the specific growth rate drops in comparison to low density cultures. The specific growth rate of exponentially growing cultures can be achieved in heterotrophic cultures under optimized conditions of temperature, light intensity, mixing and CO2 supply. Using such systems the photosynthetic efficiencies of ~7% may be achieved but it results in increased bioreactor maintenance costs thus making the process economically unviable [35].
Algae are generally cultivated in variable open- culture systems or controlled closed culture systems and both possessing their own advantages and disadvantages. The detail of each system is discussed below:
Land based open culture systems
Land based open culture system operation is limited to areas where low cost water is available due to the low depth and large surface area and water loss through evaporation can become a major issue. Marine waters and wastewaters can serve as good matches for this system, as environmental and sustainability issues would prevent large open pond cultivation using potable water. There is already some experience on large scale production using these types of systems, either in pilot projects partially funded by the government, in wastewater treatment plants, where it is used in secondary or tertiary treatment of sewage, or in commercial scale algal cultivation for the health food market [36].
Shallow unstirred ponds: Shallow and unstirred ponds are the simplest of the land-based open culture system for algae cultivation. Their sizes vary from few m2 to 2,500,000 m2 and use CO2 as carbon source. Although open-culture systems are easy to operate, less expensive and have large production capacity but uses more energy and do not allow control of temperature and lighting conditions. It is more easily prone to invasion by other algae and contamination from bacteria. Slow diffusion of nutrients, dead and living algae sedimentation, flotation and limited usage of available sunlight are some other problems that add to the misery of the open-culture systems [13,14,34,37,38].
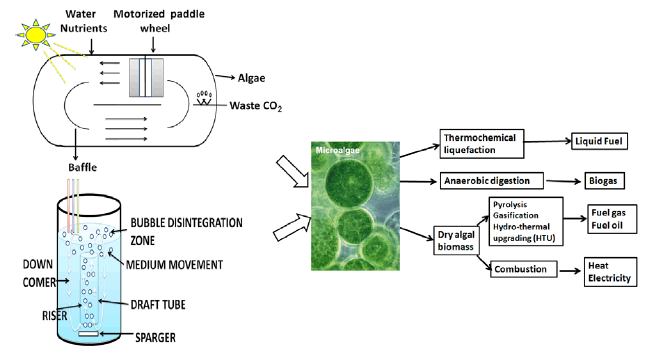
Circular/raceway ponds: The race-away ponds have tried to minimize the limitations by using mechanical agitators to provide aeration. In mechanical agitator, arm move in a circular motion, and a paddle wheel cause the circulation of water through narrow pond (Figure 2). The gas bubbles can be blown and part of this gas is used to as carbon source and rest provide medium agitation. A limited number of species can be maintained in an open system, and hence the locallyoccurring strain is preferable in an open system [13,14,34,37,38]. The outdoor commercial production of microalgae was achieved in Arthrospira, Chlorella and Dunaliella genera species only because they show high growth in selective medium (basic or highly saline) and also show reduced contamination issues. Such systems are less energy intensive, easy to operate, cheap and more durable than closed systems [39].
Closed systems
The purpose of the mass algal culture and local weather conditions may make the choice of system obvious. However, the main comparison between the two systems is principally cost and productivity. Earlier closed systems were created by covering the pond with a greenhouse. While this resulted in a smaller systems but many problems associated with pond systems were tackled. It allows more species to be cultivated and extend their growing season at will. Open ponds require larger and more cultivation areas to achieve the same productivity. The low mixing rate of open ponds intensifies the self-shading effect due to cell concentration and the physical structure of open ponds prevent proper aeration, causing a low medium CO2 partial pressure, thus limiting the productivity rate per unit of area and volume.
The growth rate can be controlled variably in closed systems. Regarding the productivity per unit area or volume, Photo-Bioreactors(PBRs) are said to outperform open ponds. Growing algae in closed bioreactor for bioenergy production is a costly affair for biofuel production. Since the single cell oil (SCO) used in pharma, health and cosmetic industry are high value product (costing €1000 s per kilo), upscaling of algae for commercial use must supply biomass for a competent production price with other renewable and nonrenewable energy raw materials. Currently only open systems can be used for large scale culturing of algae with minimal financial and energy input [34,40] but they have their pitfalls. With time many researchers attempted to create different type of bioreactors with better monitoring control to upscale the commercial production of algae.
Types of bioreactors: Photobioreactors: These mainly involve photoautotrophic production using natural or artificial lighting, although conventional stirred bioreactor can be used to culture some microalgae species heterotrophically at high densities in the dark. Photobioreactors on the other hand, are systems that are flexible and can be optimized in accordance with the biological and physiological characteristics of the species being cultivated. Thus, minimizing the contamination and offering better control over culture conditions.
Major limitation of photobioreactor is oxygen build up (due to photosynthesis) until they inhibit and poison the algae [41]. So degassing of the culture is a must and it is achieved by periodically returning the culture to a degassing zone. Carbon starvation and an increase in pH often occur as the algae use carbon dioxide. Therefore, carbon dioxide must be fed periodically into the system so as to successfully cultivate the microalgae during scale-up. Photobioreactors may require cooling during daylight hours and the temperature regulation can be achieved through heat exchangers.
Vertical column Photo bioreactors: Vertical column photo bioreactor is made up of glass or acrylic tubing which allows penetration of light for culture of algae. A gas sparger system, at the bottom of the reactor converts the inlet gas into tiny bubbles, which allows for mixing, mass transfer of CO2, and removing O2 produced during photosynthesis. Normally, no physical agitation system is implemented in the design of a vertical column photobioreactor. Vertical photobioreactors can be categorized into bubble column and airlift reactors (Figure 3) based on their liquid flow patterns inside the photobioreactors [42].
Bubble column reactors consists of cylindrical vessels with height to diameter ratio greater than 2. They are less expensive and have higher surface-area-to-volume ratio. They do not have moving parts and heat and mass transfer is maintained by gas bubbling upwards from the sparger. The efficient release of oxygen characterizes a bubble column reactor. Sparger design is important for the performance of the photobioreactor. Perforated plates used assparger for breaking and redistributing coalesced bubbles. Light is supplied from outside the column. Circulation of the liquid from central dark zone to the external light zone creates differential gas flow rate which is critical for the photosynthetic efficiency [43].
Airlift reactors are made of a vessel with two interconnecting zones. The gas mixture flows upward to the surface from the sparger in one tube, called gas riser. The other region, called the down comer, is where the medium flows down toward the bottom and circulates within the riser and the down comer. An airlift reactor based on the circulation mode is further categorized into internal loop or external loop [44]. Residence time of gas in different zones affects gas-liquid mass transfer, heat transfer, mixing, and turbulence and thus is critical for controlling the performance. A rectangular airlift photobioreactor have shown better mixing characteristics and better photosynthetic efficiency, however their design are complex and hence suffer difficulty in scale-up.
Flat Plate Photobioreactors (FPP): FPP consist of two rectangular panels made of glass or Perspex spaced about 25 mm or apart, illuminated on both sides and stirred by aeration (Figure 4). The panels may be inclined in some designs to capture optimal amount of radiations and mixing of algal culture is achieved by either by pumping or aeration systems. The aeration helps to remove the oxygen generated during photosynthesis, which is a limiting factor owing to photorespiration. Carbon dioxide can also be added to control growth whereas the temperature is controlled by spraying the water over the panel surface. Though expensive, some systems used heat exchanger for this purpose. However, flat plate systems may also suffers from some problems such as high space requirements, high solar energy, difficult maintenance, and low efficiency in terms of mass production per unit of space [45].
The productivity of FPP highly depends on the space requirements between the panels and the areal productivity constraint for outdoor application. On the other hand, flat plate systems when operated at indoor conditions, the factors such as distance between light sources and panels, temperature effects, illumination of one or both panel sides, light path are crucial. Increasing volume causes increase in hydrostatic pressure, thus making scale-up difficult. Moreover, the hydrodynamic stress may affect the microalgae growth. Though flat panels are very productive but difficulty in scaling up and high cost required to operate, limits its use [46]. Cheaper design was proposed by Rodolfi et al. where they used plastic bags within the rectangular frame [14]. The major limitations are: the scale-up require many compartments and support materials, difficulty in controlling culture temperature, some degree of wall growth and possibility of hydrodynamic stress to some algal strains.
Horizontal Tubular Bioreactors (HTB): Tubular systems are widely used commercial closed-culture systems for algal production. Tubular photo-bioreactors are made of transparent polypropylene acrylic or polyvinyl chloride pipes and have small internal diameters to increase light penetration. An air pump or airlift system is employed for mixing and agitation of the culture. They can be horizontal, vertical, inclined or conical tubular photo-bioreactors (Figure 4). Large illumination area makes them a good candidate for algal culture. However they have some limitations during scale-up. Mass transfer (oxygen build-up) and photo-inhibition becomes a major problem when we go for scale- up. Also, scale-up is done by increasing the diameter of the tubes, thus the cells at the lower part of the tube does not receive enough solar energy required for growth [41].
It is also difficult to control the temperature in the tubular bioreactors, though thermostat or cooling tubes can be used but it is expensive and difficult to implement. In long tubular photobioreactors, gradients of oxygen are formed and CO2 transfer along the tubes takes place. In HTB increase in pH of the cultures occurs which require frequent re-carbonation of the cultures, which would add to the cost of algal production. HTB also require large land space. But HTB have large illumination surface area and hence suitable for outdoor cultures. They also show fairly good biomass productivities and hence are relatively cheap [46,47]. Helical tubular bioreactor made up of teflon or low density polyethylene named ‘Biocoil’ was designed in UK and tested successfully at pilot scale (2000 L) for growth of various algae [48].
Of course PBRs have several advantages over open ponds as a cultivation system. However, an open pond is considerably cheaper. The total cost can be analyzed as infrastructure costs (CapEx), maintenance costs and operational costs (OpEx). All these factors favor open ponds. The installation and maintenance costs of PBRs may prove prohibitive for the production of low cost compounds, but acceptable for the nutraceutical industry. Production of compounds such as Carotenoids and some poly-unsaturated fatty acids (PUFAs), such as omega-3 and linoleic acid in a microalgal system, justifies the use of PBRs.
Different PBR designs are being tested and some studies have shown promising results using systems requiring only relatively simple operation and maintenance. Using innovative 110 L flat green wall photobioreactors, a production of 204 mg L-1 d-1 was reached [14]. Thus, open ponds offer a cheaper operation, but at the expense of productivity. Long term studies with outdoor open ponds have reported productivities ranging from 20 to 50 mg L-1d-1 [49,50].
‘Big-Bag’ systems
Probably the longest used closed culture systems for mass culture of microalgae are the ‘big-bag’ systems generally used in aquaculture butcheries to feed larval fish, crustaceans, mollusks or rotifers. Although widely used these systems are notorious for the instability of the culture. This instability probably occurs because mixing in these bags is uneven, leading to the build- up of the cells in unmixed areas, which in turn leads to the cell death, especially if the culture is not axenic (bacteria free). To achieve reasonably reliable cultures, it is essential to maintain axenic conditions, a feature that is not essential for the tubular photobioreactors [51].
In recent years, new technological breakthroughs led to designing of unique type of bioreactors. The green solar collector (GSC) uses Fresnel lenses that distribute and focus light efficiently inside a bioreactor [52] (Figure 4). Valcent's HDVB (high density vertical bioreactor) system is a closed circuit process and hence requires little water. Algal growth is precisely controlled by fluid mechanics, gas-liquid mass transfer, and culture is mixed using an airlift pump [53]. Colorado based Solix have develop a clear flat plate photoreactor that utilize the smokestack exhaust from power plant as carbon dioxide source, hence cutting the 90-95% production cost of algal culture [54].
CO2 enrichment: One approach for raising productivity is CO2 enrichment. To ensure CO2 fixation, the carbon concentration mechanisms (CCM), helps the cell locally increases the CO2 concentration around the Rubisco enzyme to ensure its functioning [36]. This mechanism is wide spread amongst the algae and illustrates the advantages of raising the CO2 concentration in mass cultures. Indeed, sparging CO2 into the culture medium is known to increase its cellular concentration and two different approaches are frequently reported, the use of CO2 to adjust the pH, and CO2 enrichment as a way to mitigate flue gases [14,55-57]. Of course any feedstock used in large scale production will play an important role on the final price and CO2 is not an exception. Thus, this type production should optimally be coupled to a bioremediation process.
Moreover, microalgae can effectively convert solar energy into a variety of valuable end products, such as biofuels, food additives, and compounds used in cosmetics and pharmaceuticals [58]. For example, S.obliquus [59], C. vulgaris [60], and Chlamydomonas sp. [61], exhibit both a high CO2 fixation rate and high lipid/carbohydrate content. Thus, combining microalgae-based CO2 fixation with biofuel production or bio-based chemical production seems to have a high potential for reducing the level of atmospheric CO2 while simultaneously producing biofuels or valuable chemicals.
Offshore culture of macroalgae
Macroalgae are long multi-cellular algae (measure in inches/feet) often grown in open ponds and oceans (as seaweed e.g. giant kelp plant). Macroalgae can be grown in offshore systems (i.e. algae culture in the open ocean). Macroalgae hold promise as a raw material for fuel because they produce more biomass per unit area per year. The main species relevant to biofuel production are chlorophyta (genus-Ulva and Caulerpa), red algae (Gigartinales, Halymeniales and Palmariales) and brown algae (order of Fucales, Laminariales and Tilopteridales) [62]. They can be grown on sewage, municipal waste water or agriculture or farm runoff water. In Florida scientist created algal turf scrubber (ATS) in shallow canals having nylon netting on which filamentous algae can form colonies. Studies on algal turf scrubber (ATS) has revealed that the algae can capture around 60-90% of N(nitrogen) and 70-100% of K(potassium) from manure effluents runoff water thus reducing eutrophication of water bodies. On harvesting, the macroalgae can be used as an organic fertilizer [63].
Harvesting and processing of algae
After culturing in open or closed systems, the algal biomass needs to be harvested for further processing. However, harvesting microalgal cells is quite challenging. The microalgae cannot be easily harvested as macroscopic plants, and thus the consequent oil extraction is more complicated. Moreover, algal cultures are very dilute, usually around 1% for autotrophic growth up to 10% for heterotrophic growth [64-65], thus making dewatering a necessary step prior to biomass use. In algae harvesting, dewatering is most capital and energy intensive (-30% of total cost) step. Harvesting from bioreactor is less expensive as compared to open pond system because the biomass productivity can on average be 13 times more than the open pond [34]. In open culture the biomass yield is around 0.5-1.0 gL-1, while in closed system it reach around 5-10 gL-1. Gravity settlement, filtration and centrifugation are the commonly used harvesting method [34]. At times flocculation step or flocculation-flotation is added to aid the harvesting. The choice of harvesting technique is governed by microalgal species used and final product. Uduman et al. summarized the different harvesting techniques used in algae biomass recovery (Figure 5) and concluded that centrifugation is most efficient recovery technique though the cost is high [66]. Many standard techniques have been evaluated for use in mass algal cultivation and their limitations are reviewed in detail elsewhere [33,58,67,68]. The choice of harvest method vary depending on the ultimate use of the biomass like Nutraceutical products require physical processes for harvesting, thus avoiding chemical contamination, and maintaining the product’s natural characteristics. As a result the high value of the product gets compensated for the high cost and energy intensity of the method.
Filtration under vacuum can enhance the biomass recovery but they were relatively slower. Alternatively, ultra-filtration results in good recovery, however they require high energy input and filter clogging is frequent making it unsuitable for large scale processes. Mata et al. made use of simple large unit micro strainers as an attractive alternate harvesting strategy [67]. Israel based Algatech Ltd. developed zero energy requiring harvesting step based on sedimentation for Haematococcus (efficiency>90%). In recent years, electrocoagulation electroflotation and Electro Coagulation (EC) are emerging as cost effective and efficient harvesting systems and have been used for harvesting of Chlorella vulgaris, Phaeodactylum tricornutum and Nannochloropsis sp. with good quality biomass [69-71].
Microalgal harvesting cost can be reduced by harvesting cells by flocculation. In alkaline flocculation, flocculants counterbalance the repelling surface charges on the cells hence cause the algal cells to coalesce to form floc. In such method the flocculant needed in a linear stoichiometeric fashion hence making process very expensive. Schlesinger et al. reported that major hurdle to bulk algal production and harvesting can be tackled by non-toxic inexpensive alkaline flocculation. It is observed that the flocculent amount is governed by a function of logarithm of cell density and in dense cultures like biodigestable sludge, it should reduce the cost of the flocculation below $1.00 per 1000 kg of algal biomass [72].
Strain selection, culturing and harvesting
With existing technology, it is obvious photobioreactors are simply too expensive and for a low value product such as a biofuel open ponds must be used. A simple calculation based on possible solar energy inputs and maximum photosynthetic conversion efficiencies shows that the resulting energy value per square meter allows very little capital expenditure for cultivation facilities. Therefore, although specific laboratory strains have shown to produce high levels of lipids, i.e. Botryococcos braunii, but they cannot be used in practice in an open system as there are chances of overrun by indigenous species. Cost effective harvesting is of course a major unsolved challenge, and several new advances have already been discussed in the manuscript [36].
Biofilms
A very little research has gone into to study species that readily form biofilms for biofuels production due to difficulty in maintaining their homogenous suspension cultivation medium. However, several recent studies, with two different systems, have shown that this kind of growth mode can offer the ease of simple mechanical harvesting, leading to slurries with a dry weight content of 9-16%. In one case, algae were grown on a rotating drum imitating an open pond system where harvesting was achieved by simply unspooling and scraping the cotton rope fiber that was used [73]. Another approach, a flat surface which was drip-watered was used to grow algae and were recovered by simple mechanical scraping at the end of growth period. In both cases, not only harvesting method was simplified but the researchers were also able to achieve high rates of biomass production at respectable light conversion efficiencies.
Pretreatment strategy
Microalgal pretreatment is prerequisite for separation of its different useful components, which are further processed to different types of biofuel. Carbohydrate of algal biomass is converted either to ethanol or biogas using fermentation. The biomass is processed in three sequential steps-hydrolysis, acidification and energy generation. Out of these steps hydrolysis is often observed as a rate limiting step. The rigid cell wall and membrane make them resistant to biodegradation or show slower biodegradation during fermentation. For algal oil harvesting expellers/presses, solvent extraction and supercritical CO2 are conventionally used. For algae having high oil content expeller/ pressers are used which mechanically rupture the algal cell (70-75% recovery) [74].
In solvent extraction benzene, ether and hexane are employed to degrade cell walls. Using distillation, oil is recovered from the solvent and this step can be coupled to expellers step for efficient recovery of the oil from biomass (~95%). Supercritical CO2 penetrated algal cells at high temperature and pressure and cause its rupture (~100%). Supercritical CO2 treatment are costly, energy intensive, highly sensitive and involves risk due to high pressure requirement [75].
Numerous studies on pretreatment are undertaken including ultrasonication, bead beating, thermochemical, osmotic shock, nano dispersion, enzymatic and microwave treatment [76,77]. Ultrasonication does not require high temperature (like autoclaving and microwave) and expensive chemicals and is therefore most economical [78]. Cell disruption in ultrasonication is achieved either by microbubble cavitation or acoustic streaming [79]. Choi et al. reported enhanced microalgal cell wall disintegration which resulted into increased ethanol and hydrogen production [80]. Grinding of algal biomass resulted in doubling of yield of algal fatty acids extraction. Nano dispersion of crude algal biomass resulted in 62% enhanced yield of total extracted fatty acids [76]. Bremauntz et al. reported that osmotic shock (0.1 M NaCl) pretreatment for 24 h resulted in approx. 30 times increase in fermentable sugar in the Scenedesmus sp. Strain [81]. In case of Botryococcus braunii osmotic pretreatment enhanced the recovery rate of hydrocarbon >90% than that of untreated sample [48]. Passos et al. investigated the microwave pretreatment on biogas production using microalgae and concluded that microwave pretreatment improved the dissolution and digestibility of microalgae [82]. In enzymatic pretreatment cellulases and lipases are generally used. Enhanced biogas production was achieved when Botryococcus braunii and Nannochloropsis gaditana were pretreated with bacterial strains having endoglucanase/amylase and cellulase activity [83].
Origin Oil Inc. reported (2010) first single-step extraction of algal oil technology, by using quantum fracturing in combination with electromagnetism and pH modification. After cell rupture algae oil rise and float on the top and removed for skimming and refining. The degraded biomass settles to the bottom of the extraction tank. In this technology, CO2 injection are used to lowers pH and quantum fracturing cause mechanically distresses in algae cells. Fine-tuned electromagnetic field is then used simultaneously to rupture algae cells. This breakthrough technology can be fine-tuned for range of feedstock and it may result in highly scalable, cost effective oil harvesting system for biofuel generation. Origin Oil Inc. has also innovated and scaled up (up to 200 gallon tank) a technology known as “Live Extraction”, where oil is harvested from electrically stimulated live algae continuously [84].
Ames Lab of US Department of Energy also develop a process based on nanospheres for extracting the fuel relevant chemicals Ames lab program director Victor Lin in collaboration with Catilin Inc. and Iowa State University-Center for Catalysis have developed mesoporous nanoparticles which can selectively extract fuel-relevant chemicals from the algal lipid without killing them. These compounds (free fatty acids (FFA) and triglycerides) are converted to biodiesel using commercial T300 catalyst of Catilin. Cavitation Technologies Inc. (CTI) used nanoreactor to create cavitation bubbles in a solvent material and collapse of these bubbles near algal cell wall forms shock waves and liquid jets that results in the cell wall breakage and ultimately cause the contents release into the solvent [85].
Upgrading technology
After harvesting, pretreatment and separation of components, each is converted to suitable bioenergy forms like biodiesel, biogasoline, ethanol, butanol, biohydrogen etc. [86]. PNNL (Pacific Northwest National Laboratory) researchers have developed continuous chemical processes which produce crude oil in less than an hour, and Utahbased Genifuel Corp. got the license for the technology and building pilot plant based on PNNL process. In Brazil, Solazyme Bunge ProdutosRanovaveis Ltd. developed microbial dark fermentation based commercial close bioreactor based plant for conversion of cane sugar to ‘tailored oil’. Solazyme in partnership with Chevron has been awarded a grant of $21.8 m from DoE for development of algal fuel demonstration plant for US Navy trails. SoladieselTM emerged as a superior to ASTM D6751 forjet fuel, D-975 and military specification and clean diesel technology [87].
After oil extraction, the green crude is further subjected to trans-esterification reaction. The green crude is mixed with sodium hydroxide (which act as a catalyst) and alcohol and results in fatty acid methyl esters (FAMEs/biodiesel) and glycerol. The mixture is processed using hydrocracking and hydrogenation and glycerol (byproduct) is removed and pure algal biodiesel is harvested and termed as 'Green diesel'.
In recent years hydothermal liquefaction (HTL) has replaced the conventional transesterification method for the production of biodiesel. In this process, elevated temperatures (250-250°C) and pressures (10-20 Mpa) are applied to high moisture algal biomass to break down and reform the chemical molecules into green-crude oil. At such high temperatures and pressure, water acts as a highly reactive medium and promotes the breakdown of chemical bonds, leading to reformation of biomolecules, thus mimicking the natural geological process which produces our current fossil fuels. These conditions make HTL well suited for the conversion of a wider range of feed stocks like low lipid algae, fast-growing algae that proliferate in wastewater treatment facilities, swine manure, garbage and sewage waste. Thus aiding in reducing environmental pollution, producing bioenergy and preserving valuable water resources. The resulting green-crude oil can have combustion value comparable to crude oil, depending upon the feedstock. It can be further burned in boilers or upgraded to higher value fuel [88,89].
Algal biomass can be converted to variety of fuels using ABE (acetone, butane, and ethanol) Process of Charles Weizmann [90]. In this process the sterilized biomass is fermented with Clostridium acetobutylicum at 35-36°C for 58 hours. Fermentation starts after 5-10 hours of lag phase and completes in 48 h, after which mixture is fractal distillated to separate each component. Biobutanol is less energy dense (10%) than gasoline (energy value 114, 000 BTU/Gal) and has higher energy value (110,000 BTU/Gal) as compared ethanol (76,100 BTU/Gal) or methanol (Green Biologics, Website). In most engines, butanol can be used as alternate to gasoline and does not require any modification. It can be blend with gasoline (at 20%), diesel (at 40%) and result in better performance and provide resistance to corrosion than E85 [91]. Bioethanol is another alcohol based biofuel and a Florida based company Algenol developed a breakthrough DIRECT TO ETHANOL® technology which utilize sunlight, algae, non-arable land/ sea water and carbon dioxide/industrial exhaust to produce ethanol and algal biomass. Porphyridium cruentum accumulates higher amount of carbohydrates hence have potential as a suitable raw material for ethanol production [92].
Artificial light can be provided by any regular light source such as tungsten or fluorescent bulbs. Low heat generation, the specificity of the wavelength of emitted light, low power consumption and, allowing the restriction of light to photosynthetic active radiation, the influence of different wavelengths and intensities on these microorganisms has led to the use of LEDs. A recent study showed that different wavelengths may have a significant influence on biomass and lipid productivity, as well as on the lipid profile. A strain of Nannochloropsis showed a higher growth rate, lipid productivity and different lipid profile under blue light (470 nm) when compared with growth under red (680 nm) or green (550 nm) [93].
Bio-gasoline is produced by directly converting sugar into gasoline using APR (aqueous phase reforming) technique. The carbohydrate solution containing alcohol, glycerol, cellulosic sugar, starch, liquid phase reforming single reactor system at sugar alcohol is fed into temperature of 453.15-538.15 K and 6,800,000 Pa pressure and converted to mixture of oxygenated hydrocarbons, which is further processed using conventional chemical processing to non-oxygenated hydrocarbons. Biogasoline (BG100/100% bio-gasoline) resembles gasoline in its components usually containing hexane and dodecane atoms per molecule and hence can be used as a substitute to gasoline in internal-combustion engines [94]. Owing to its chemical similarity, it can blended with regular gasoline. Though small percentage of octane booster is required by bio-gasoline to match conventional gasoline. In the year 2010, the first bio-gasoline demonstration plant was set up in Madison, WI by Virent Energy Systems, Inc.
Methane is another biofuel that can be produced using algal biomass. Commercial production by either by gasification production of methane is achieved, pyrolysis under high temperature and pressure or by anaerobic digestion.
Economical aspect
The estimation of economic viability of algae based biofuel is a daunting task, primarily because commercial production of algae for bioenergy is not fully explored yet. Secondly the other algal products tend to have a ten times greater value than biofuel (Figure 6). The production system needs to be subjected to a detailed LCA (life cycle assessment), to determine possible environmental impacts, NER (net energy ratio), and an economic analysis before going on for large scale microalgal biofuels production. However, to do this in a meaningful way requires specific inputs on system components, and since many of the outstanding questions raised here; cultivation method (open ponds versus photobioreactors), harvesting technologies, and even extraction and transesterification reactions, remain to be answered, this cannot really be done in a meaningful way at present. An economic analysis which compares the price at the pump of a biofuel with that of a fossil fuel is in fact wrong. A metaeconomic analysis for biofuels is one that takes into consideration indirect costs associated with fossil fuel production and use. A quick overview suggests that there are in fact many hidden costs to fossil fuel use and that the ‘‘real’’ cost of gasoline or diesel is significantly higher than the price paid by the consumer at the pump.
One way to estimate the damage is to look at the cost of adapting to climate change, although this does not provide the actual full costs incurred since this represents less than full mitigation. An initial international study estimated these costs at $49 to 171 billion (USD) per year (UNFCCC, 2007) and it has been argued that this is in fact an underestimate [94]. Of course, these estimates are highly dependent on the accumulated of atmospheric CO2 burden over time as well as a great deal of uncertainty as to actual impacts. Thus, determining what the competitive cost of a biofuel really should be will require detailed economic analysis. In addition, as mentioned above, detailed costing is not possible given the many uncertainties in the design specifics of a practical algal biodiesel plant. Thus, a realistic cost analysis is impossible at present.
The commercial importance of Microalgae is an untapped resource. Thus production of biogas, biodiesel and other co-products (viz. beta-carotene, PUFA, biofertilizers, among others) can be more environments sustainable and profitable if complemented with wastewater and flue gas treatment. Moreover, Microalgae biomass is also marketed in tablet or powder formulations to be used as food additives in the health food market (e.g. Arthrospira, Chlorella, etc.). Algal biomass is also used for preparation of animal feed supplements and aqua culturing (e.g. Fish feed). Economic viability of commercial algal biofuel production can be achieved if beside fuels rest of high value added products are co-produced via using an integrated biorefinery [95,96].
The capital investment in culturing microalgae and algal oil production costs range from $10.87 gallon-1 to $13.32 gallon-1 [97]. So culturing algae for bioenergy or biofuel production must be clubbed with some high value co-products to make process more economically viable. Researchers are exploring new opportunities in culturing algae in low economic value (e.g. waste land or waste streams) which does not require stringent condition (and capital) and can be used as efficient system to treat waste streams and also decrease greenhouse gas emission.
Using conventional method energy cost per kg of crude oil is estimated to be 1.24 $/kg while due to single step extraction strategy developed by Origin Oil the energy cost per kg of crude oil dropped to 16.4% of the original cost (0.20 $/kg ).
Challenges
Despite of technological advancements, commercial production of algal biofuel is still too costly because of following factors: cultivation system design requires temperature and growth limiting condition control (CO2, H2O sources, energy, nitrogen and phosphorous, sitting and resources) which adds to the cost of production and invasion, instability and fouling of culture results in lower yield of biomass. Algal growth in culture is dilute and therefore requires dewatering before further processing, which is currently an energy intensive and expensive step. After harvesting, algal biomass is processed to carbohydrate (bio-ethanol), lipid (gasoline, biodiesel, jet fuel) and protein (power generation) fractions. Fractionation, extraction technologies and product purification steps used in processing are required to be improved in terms of price, energy input, and scalability.
Biodiesel pose major challenge in terms of cold weather operation and stability. Other challenges are fuel’s compatibility with current automobile engine or infrastructure, market share and pricing and coproducts market demand. Space management for biomass, fuels, and products handling and storage is an overwhelming issue. Government and industry regulations and standards should be met in terms of GHG emission and pricing.
Current status of microalgae bioenergy in some countries
In November 2006, the U.S. Green Energy Technology Company and Arizona Public Service Company co-operated in November 2006 for the establishment of a microalgae production system in Arizona State. It connected flue gas which contains CO2 to culture microalgae in a large-scale and finally convert it to biofuel. The yield of biofuel reached 5,000-10,000 gallon per acre per year [98]. In 2010, $ 6 million funds were allocated to the Arizona State University by the Department of Energy in the U.S. to establish Sustainable Algal Biofuel Consortium (SABC). The U.S. government financially supported many other projects like creating Algae Biofuel Commercialization Consortium (CABC) and Cellana LLC consortium of Kailua-Kona and Hawaii [98].
Countries other than U.S have also started paying attention to microalgae bioenergy development. Chinese Academy of Sciences has successfully developed a large “S” shaped pipe of closed photo biological reactor. Shandong University of Science and Technology, China in 2008 used the thermal power plant and chemical plant flue gas to supply microalgae cultivation CO2 in the tower dimensional cultivation reactor and finally culture microalgae were used for producing bio-oil. The new Austrian technology company is brewing a huge plan. They invested to microalgae ecological base in the Daqi of Inner Mongolia. 280 hectares of microalgae base has been completed in 2013. Microalgae ecological base will be officially reached industrialization in 2014 [99].
In 2007, Umeå Energi was set to build an algae production plant in the north part of Sweden, headed by Swedish University of Agricultural Science, and the development funding from Umeå Energi, Umeva AB, Ragn-Sells AB and Energi myndigheten. The wastewater reclamation of algae production process, valuable algae biomass production, and CO2 sequestration from flue gas are the focusing areas under this project [100]. In September 6, 2011, the EU has launched the Algae Development Project (EnAlgae) which focused on the growth and yield information of microalgae and giant algae growing in the North Western Europe.
Microalgae emerged as an ideal bio renewable resource for biofuel production that eventually could replace petroleum-based fuel. Currently, algal based biofuel production is not commercially viable due to the capital intensive bioreactor and post harvesting steps and variable biomass productivity. The algal biofuel production can be economical either by integrated bio refining of high value products or by enhancing biomass/oil productivity by recombinant DNA technology, algal biology and engineering culture system. It is important to develop engineering strategies and optimize them to significantly improve feasibility of microalgae-based biofuel. Other than biofuel, algae find wide applications in various domains e.g. algal biomass after removal of oil can be used as nutrient rich fertilizer (e.g. ATS). It can be used in wastewater-treatment facilities to clean and purify water with little amount of chemicals. Algae can be used as a pollution control agent as they metabolize CO2 and release oxygen, hence reduces carbon emissions significantly.