Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Research Article - (2022)Volume 10, Issue 6
Pakistan is one of the most densely populated countries of the South-East Asian region, and rare hematological malignancies like MPNs are not uncommon, mainly attributable to the diverse ethnicity of the population which is endowed with discrete clinical and molecular characteristics. Being a low-middle income country, the scarcity of health care resources cause a delay in referrals to tertiary care hospitals especially from remote areas consequently leading to delayed diagnoses and management, resulting in dismal outcome of patients. National Institute of Blood Diseases & Bone Marrow Transplantation is the largest tertiary care hematology center where patients from all the four major provinces of Pakistan are referred for treatment. We prospectively studied the incidence, clinical behavior, treatment response, and challenges in the management of patients with CALR mutated ET and PMF in Pakistani population. The aim was to increase the understanding and knowledge of the treating physicians regarding CALR mutated MPNs across the country as well as to add to this rising body of international literature through a detailed clinicopathologic analysis of 218 MPNs.
CALR mutation was detected in 37.93% of ET and 37.25% of PMF, whereas Janus Kinase 2 (JAK2) mutation was detected in 50% of ET and 53.92% of PMF patients. 12.06% of ET and 8.82% of PMF patients were triple-negative. All CALR mutated patients received hydroxyurea as first-line agent. 45% and 31.6% patients in CALR mutated ET and PMF group respectively were switched to second line agent, interferon alpha to achieve a clinical response and significant reduction in the mean platelet count. The OS among ET and PMF patients was statistically similar in all three mutational groups. We conclude that CALR mutation analysis is crucial for the diagnosis of JAK2 negative MPN in our population as it poses clinical challenges for treating physicians due to distinct clinical characteristics and is associated with refractoriness and resistance to first line agents in considerable number of patients, though the overall survival of patients remains unchanged.
CALR mutation; JAK2 mutation; Essential thrombocythemia; Primary myelofibrosis; Myeloproliferative neoplasms
In late 2013, Klampfl et al, [1] reported somatic mutations in Calreticulin (CALR), the gene which encodes endoplasmic reticulum chaperone protein calreticulin, in approximately 60%-90% of JAK2 unmutated MPN patients. Type-1 (L367fs*46) and type-2 (K385fs*47) CALR mutations are the most frequent [2]. New insights have revealed that CALR mutated ET and PMF carry distinct clinical phenotype and prognosis. The presence of CALR mutation is associated with younger age, more severe degree of anemia, higher Total Leucocyte Count (TLC) and platelet counts, lower DIPSS-plus scores, and better survival compared to subjects with JAK2 mutations [3]. Some recent data suggest that CALR mutations may only have a favorable prognostic impact in subjects with type-1 mutations [4]. The prognostic impact of CALR mutations was first suggested by Klampfl et al, [1] who found, in a retrospective analysis, that patients with PMF harboring CALR mutations had significantly better overall survival compared with either JAK2 or MPL mutated subjects, with a similar trend reported in ET. CALR mutated ET patients were also found to be at lower risk of thrombosis. Nangalia et al, [5] reported that CALR mutated ET patients presented with significantly higher platelet counts and lower hemoglobin than JAK2 mutated counterparts. Findings in ET patients have subsequently been validated in two independent series [6]. CALR subtypes have also displayed an impact on the clinical phenotype and outcome of ET and PMF. In PMF Type 1 CALR mutations are more prevalent then type 2, while in ET, they are more balanced [7].
Most of the evidence regarding the disease biology and therapy of patients with CALR mutated MPN comes from US and European countries. Recently European leukemia net has published a consensus-based proposal for the management of unmet clinical needs in ET. Certain clinical aspects of CALR mutated ET were addressed including platelet therapy in patients at low risk, management of extreme thrombocytosis in low risk patients and when to initiate cytoreductive therapy in these patients [8]. There is limited evidence available regarding the clinicopathologic features and management of patients with CALR mutant MPN from the low middle income countries of South East Asian region. It has been reported previously that race and ethnicity influences the disease phenotype and clinical behavior in MPN [9].
Pakistan is one of the most densely populated countries of the South-East Asian region, and rare hematological malignancies like MPNs are not uncommon, mainly attributable to the diverse ethnicity of the population which is endowed with discrete clinical and molecular characteristics. We studied the incidence, clinical behavior, treatment response, and challenges in the management of patients with CALR mutated ET and PMF in Pakistani population. The aim was to increase the understanding and knowledge of the treating physicians for CALR mutated MPNs in Pakistan as well as to add to this rising body of international literature through a detailed clinicopathologic analysis of 218 MPNs.
Study design
It was a prospective observational study conducted at National Institute of Blood Diseases & Bone Marrow Transplantation between 2014 and 2019. The study was approved by the ethics committee of NIBD & BMT (NIBD/RD-IRB#141/10-2014). Informed written consent was obtained from all patients.
Diagnosis and risk stratification
The diagnosis of ET and PMF was based on World Health Organization (WHO) classification of Myeloid and Lymphoid Malignancies 2008. Samples for molecular analysis were collected at baseline. Symptom-assessment scoring was done at baseline and at follow up visits to document changes in constitutional symptoms before and after treatment. Liver and spleen sizes were measured in all patients. All ET patients were categorized into high, intermediate and low risk based on the IPSET score [10]. DIPSS plus scoring system was used to categorize PMF patients into low, intermediate-1, intermediate-2 and high-risk groups. Risk variables included age ≥ 60 years, hemoglobin ≤ 10 gm/dl, TLC count ≥ 25 × 109/L, presence of constitutional symptoms and circulating blasts [11].
CALR mutation analysis
DNA extraction was done on peripheral blood or bone marrow samples collected in EDTA tubes. DNA was extracted using the MagNA Pure LC DNA Isolation Kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s instructions. Molecular analysis was performed in all patients using primers for JAK2, CALR and MPL mutation. Exon 9 of CALR was amplified using the following primers: F,5´-GAGGAGTTTGGCAA CGAGAC-3´and R,5´-AACCAAAATCCACCCCAAAT-3´. PCR was performed using 25 to 100 ng genomic DNA in 100μL PCR solution (10μL of 10 × MG Taq-HF buffer, 0.2 μmol/L of each primer, 10 μL of 2 mmol/L MG dNTPs mixture, 1 μL of MG Taq-HF polymerase [Macrogen, Seoul, Korea], and distilled water). The PCR used the following cycle protocol: An initial 5-minute denaturation step at 94°C followed by 35 cycles of 94°C for 30 seconds, 58°C to 64°C for 30 seconds (depending on the primers), and 72°C for 60 seconds, with a final 7-minute extension at 72°C. The PCR products were purified and sequenced using a Big Dye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) and an ABI 3730 XL automatic sequencer (Applied Biosystems) using the above described primers. The JAK2V617F mutation was assessed using a Polymerase Chain Reaction (PCR)-based amplification refractory mutation system [12]. The MPL W515L/K exon 10 was assessed by Sanger sequencing.
Treatment and response monitoring
The Myeloproliferative Neoplasm Symptom Assessment Form Total Symptom Score (MPN-SAF TSS) was used to assess the symptom-burden of all patients and the mean score for 10 symptoms including fatigue, concentration, early satiety, inactivity, night sweats, itching, bone pain, abdominal discomfort, weight loss, and fever was calculated [13]. Response to therapy was assessed according to revised-response criteria proposed by IWG-MRT [14]. All ET patients with no known contraindication received antiplatelet agent. Therapeutic plateletpheresis was required in high-risk ET patients who presented with thrombo-embolic manifestations or with a platelet count of ≥ 1500 × 109/L. Hydroxyurea was used as first line cytoreductive therapy followed by interferon alpha in patients, refractory or resistant to hydroxyurea. Erythropoietin and synthetic androgens were used to treat anemia in PMF patients. Ruxolitinib, a JAK2 inhibitor was offered to patients with intermediate-2 and high-risk PMF and post-ET PMF. Normalization of blood counts and age-adjusted normo-cellularity of bone marrow, resolution of constitutional symptoms and hepato-splenomegaly after a treatment of at least ≥ 12 ± weeks was considered as response to therapy in PMF [15].
Statistical analysis
Data was entered in SPSS version 21 for statistical analysis. Frequencies and percentages were computed for categorical variables. Mean ± standard deviation, median and inter-quartile range was reported depending on assumption of normality. Assumption of normality was assessed with Shapiro-Wilk test. Chi-square or Fisher-exact test was applied to compare categorical variables among study groups. Kruskal-Wallis test was applied to compare non-normal numerical variables among three mutational groups. Paired t-test or Wilcoxon sign test was applied to assess changes in platelet count before and after therapy. MacNemar’s test was applied to ascertain if patients had achieved response after therapy. Kaplan-Meier curves constructed to estimate survival rates which were compared among different groups using Log-rank test. A two tailed p-value<0.05 was taken as statistically significant throughout the study.
The disease characteristics of ET and PMF patients are summarized in Table 1. Among 218 patients analyzed in this study, 53.2% (n=116) patients were diagnosed as ET and 46.7% (n=102) patients as PMF. Overall frequency of CALR and JAK2 mutations were 37.61% (82/218) and 51.83% (113/218) respectively. Small subsets of patients, 10.55% (23/218) were TN for all three mutations. The prevalence of CALR mutation was balanced between ET and PMF patients i.e. 37.93% (44/116) and 37.25% (38/102) in ET and PMF respectively. All the CALR mutations detected were heterozygous frame shift mutations in exon 9 of CALR gene. Type 1 CALR mutations were prevalent in both ET and PMF (63.6% (28/44) and 55.2% (21/38) respectively) as compared to the type 2 CALR mutations which were detected in 36.36% (16/44) of ET and 44.73% (17/38) of PMF patients. Homozygous CALR mutation was detected in one patient with fibrotic phase of PMF, which was a unique finding, rarely reported in MPN patients previously. Among patients with different ethnic origins coming from all the major provinces of Pakistan including Sindh, Punjab, Baluchistan and Khaiber Phakhtunkhwah, CALR mutated and TN patients were mainly of Sindhi origin (45.5% and 50% respectively) whereas JAK2 mutated patients mostly had Baluchi origin (41%).
| Essential Thrombocytopenia (ET) | Primary Myelofibrosis (PMF) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients (n) | CALR (44) | JAK2 (58) | TN (14) | P-value | CALR (38) | JAK2 (55) | TN (9) | P-value | |||
| Males (%) | 30 (68.2) | 29 (50) | 6 (42.9) | 0.106 | 24 (63.2) | 28 (50.9) | 3 (33.3) | 0.108 | |||
| Age (IQR) | 36 (31-43) | 54 (34-85) | 32 (18-75) | **<0.001 | 40 (37-51) | 52 (21-76) | 43 (22-60) | *0.022 | |||
| Hb (g/dL) | 10.8 (9-15) | 12.1 (10.2-15.1) | 12.9 (11.7-16.8) | **<0.001 | 10.7 (9.3-13.4) | 9.4 (6.8-15) | 7.9 (6.5-14.2) | **<0.001 | |||
| TLC x109/L | 9.9 (5.1-16.5) | 10.7 (4.7-14.7) | 11.9 (10.8-14.7) | *0.010 | 13.1 (5.6-63.2) | 19.1 (11.9-25) | 17 (10.9-25) | **0.009 | |||
| Platelet x109/L | 1228.5 (462-2305) | 965 (92-1883) | 996.5 (382-1841) | **0.003 | 629 (12-1147) | 136 (99-196) | 248 (38-483) | **<0.001 | |||
| Splenomegaly (%) | 29 (65.9) | 36 (62.1) | 8 (57.1) | 0.861 | 23 (60.5) | 21 (38.2) | 3 (33.3) | 0.08 | |||
| Constitutional symptoms (%) | 29 (65.9) | 31 (53.4) | 4 (28.6) | *0.047 | 27 (71.1) | 31 (56.4) | 8 (88.9) | 0.098 | |||
| Haemorrhage (%) | 3 (6.8) | 2 (3.4) | 1 (7.1) | F0.589 | 2 (5.3) | 1 (1.8) | 1 (11.1) | 0.291 | |||
| Thrombosis (%) | 2 (4.5) | 4 (6.9) | 1 (7.1) | *0.011 | 1 (2.6) | 7 (12.7) | 0 (0) | 0.180 | |||
| Microvascular symptoms (%) | 30 (68.2) | 30 (51.7) | 8 (57.1) | 0.246 | 14 (36.8) | 19 (34.5) | 2 (22.2) | 0.707 | |||
| IPSET | low | 30 (68.2) | 1 (1.7) | 13 (92.9) | **<0.001 | D-IPSS | Low | 7 (18.4) | 0 (0) | 0 (0) | **0.001 |
| Int | 5 (11.4) | 41 (70.7) | 0 (0) | Int-1 | 7 (10.5) | 15 (27.3) | 0 (0) | ||||
| High | 9 (20.5) | 16 (27.6) | 1 (7.1) | Int-2 | 16 (42.1) | 28 (50.9) | 9 (100) | ||||
| High | 11 (28.9) | 12 (21.8) | 0 (0) | ||||||||
Table1: Disease characteristics of ET and PMF patients based on molecular mutational profile.
The CALR mutated ET and PMF were associated with younger median ages, 36 (IQR, 31-43) and 40 (IQR, 37-51) years, male predominance, 68.2% (30/44) and 63.2% (24/38) and higher mean platelet counts at baseline (1228 × 109/L and 629 × 109/L) respectively. Hemoglobin levels in CALR mutated ET were lower (10.8 g/dl) as compared to JAK2 mutated (12.1 g/dl) and TN ET (12.9 g/dl). On the contrary, CALR mutated PMF showed relatively higher hemoglobin (10.7 g/dl) as compared to JAK2 mutated (9.4 g/dl) and TN PMF (7.9 g/dl). Thromboembolic events were found in 4.5% (2/44) and 2.6% (1/38) of CALR mutated ET and PMF vs. 6.9% (4/58) and 12.7% (7/55) of JAK2 mutated ET and PMF with a significant statistical difference in ET group (p=0.011). Thromboembolic events were rare in TN groups. Microvascular symptoms occurred in 68.2% (30/44) and 36.8% (14/38) of CALR mutated ET and PMF versus 51.7% (30/58) and 34.5% (19/55) JAK2 mutated and 57.0% (8/14) and 22.0% (2/9) of TN groups with a p value of 0.24. Splenomegaly was found in 65.9% (29/44) and 60.5% (23/38) of CALR mutated ET and PMF vs. 62.1% (36/58) and 38.2% (21/55) of JAK 2 and 57.1% (8/14) and 33.3% (3/9) of TN groups.
According to IPSET scoring, patients with CALR mutated ET were mainly low-risk, 68.2% (30/44) and no leukemic transformation was observed in this group till the last follow up. On the other hand, DIPSS scoring for CALR mutated PMF showed majority of patients in intermediate-2 risk group, 42.1% (16/38). Leukemic transformation occurred in 5.3% (2/38), 3.6% (2/55), and 11.1% (1/9) of CALR mutated, JAK2 mutated and TN PMF patients respectively. Table 2 demonstrates the clinical characteristics of patients with type 1 and type 2 CALR mutations among ET and PMF. Among ET, type 1 CALR mutation displayed younger age versus type 2 CALR, whereas in PMF, type 2 CALR was associated with younger age. There was no significant difference in the mean hemoglobin of type 1 versus type 2 CALR in both ET and PMF. Type 2 CALR was associated with higher TLC count in PMF and higher Platelet count in ET. Splenomegaly was frequently associated with Type 1 CALR mutated ET as compared to PMF.
| Essential Thrombocytopenia (ET) | Primary Myelofibrosis (PMF) | |||||
|---|---|---|---|---|---|---|
| No of patient’s n (%) | TYPE 1 CALR 28 (63.6%) |
TYPE 2 CALR 16 (36.3%) |
P-value | TYPE 1 CALR 21 (55.2%) |
TYPE 2 CALR 17 (44.7%) |
P-value |
| Age (Range) | 33 (28-45) | 46.5 (31-73) | **<0.001 | 49 (22-70) | 39 (37-40) | **0.001 |
| Males (%) | 18 (64.3) | 12 (75) | 0.463 | 13 (61.9) | 11 (64.7) | 0.859 |
| Hb (g/dL) | 10.6 (9-15) | 10.7 (9.1-14) | 0.678 | 10.7 (9.3-13.4) | 10.5 (9.7-13.2) | 0.931 |
| TLC × 109/L | 9.7 (5.1-16.5) | 10.4 (8.9-16.1) | 0.085 | 10.9 (5.6-37) | 17 (8.1-63.2) | 0.064 |
| Platelet × 109/L | 1016 (462-1998) | 1316.5 (963-2305) | *0.030 | 597 (112-673) | 698 (12-1147) | *0.039 |
| Splenomegaly (%) | 18 (64.3) | 9 (56.2) | 0.764 | 14 (66.7) | 9 (52.9) | 0.389 |
| Microvascular symptoms (%) | 18 (64.3) | 12 (75) | 0.463 | 6 (28.6) | 8 (47.1) | 0.240 |
| Constitutional symptoms (%) | 16 (57.1) | 13 (81.2) | 0.105 | 14 (66.7) | 13 (76.5) | 0.721 |
Table 2: Patient characteristics of ET & PMF based on Type 1 & Type 2 CALR mutation.
The response to treatment with hydroxyurea and interferon in CALR mutated ET and PMF was assessed by improvement in the MPN-SAF score, reduction in platelet count and spleen size before and after therapy as shown in Table 3A and 3B. Almost all patients were symptomatic for each item in MPN-SAF score with variable intensity and required treatment with one or two agents to control constitutional or microvascular symptoms and for extreme thrombocytosis in some cases. Among CALR mutated ET, fatigue (18%) and inactivity (18%) had the highest incidence, whereas in PMF, fatigue (47%), fever (42%), night sweats (40%) and bone pains (37%) carried the highest incidence. Treatment with first line agent, hydroxyurea did not significantly reduce the mean MPN-SAF score in ET and MF patients and majority of patients in both the groups were switched to second line agent, interferon as shown in Tables 3A and 3B. Similarly, 45.5% and 31.6% patients in CALR mutated ET and PMF group respectively were switched to second line agent, interferon alpha to achieve >50% reduction in the mean platelet count as depicted in Figures 1A and 1B. The OS among ET and PMF patients was statistically similar in all three mutational groups (91% (67-98%) in CALR, 96% (77-99.5%) in JAK2, 100% in TN PMF and 92% (77-97%), in CALR, 95% (83-99%) in JAK2, 100% in TN ET).
| Symptoms | N | Baseline | POST HU | Post HU and INF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median (IQR) | SD | Incidence | Mean | Median (IQR) | SD | Incidence | Mean | Median (IQR) | SD | Incidence | ||
| Fatigue | 44 | 4 | 4 (3-5) | 1 | 18% | 3 | 3 (2-4) | 1 | 14% | 2 | 2 (1-0) | 1 | 7% |
| Early satiety | 44 | 3 | 3 (3-4) | 1 | 11% | 2 | 2 (2-3) | 1 | 9% | 2 | 1.5 (1-0) | 1 | 5% |
| Abdominal pain | 44 | 4 | 4 (3-5) | 1 | 14% | 4 | 4 (4-5) | 1 | 18% | 1 | 1 (1-0) | 1 | 7% |
| Restlessness | 44 | 3 | 3.5 (2-4) | 1 | 18% | 3 | 3 (2-3) | 1 | 17% | 1 | 1 (1-1) | 0 | 9% |
| Lack of Concentration | 44 | 2 | 2 (2-0) | 1 | 7% | 2 | 1.5 (1-0) | 1 | 5% | 1 | 1 (1-1) | 0 | 5% |
| Night sweating | 44 | 2 | 2 (2-3) | 1 | 11% | 2 | 2 (1-2) | 1 | 9% | 1 | 1 (1-1) | 0 | 9% |
| Itching | 44 | 5 | 5 (5-6) | 1 | 9% | 5 | 4.5 (4-0) | 1 | 5% | 1 | 1 (1-0) | 1 | 7% |
| Bone pain | 44 | 2 | 1.5 (1-0) | 1 | 5% | 2 | 2 (2-2) | 1 | 14% | 1 | 1 (1-1) | 0 | 7% |
| Fever | 44 | 3 | 3.5 (2-4) | 1 | 9% | 3 | 3 (2-3) | 1 | 16% | 1 | 1 (1-2) | 11% | |
| Weight loss | 44 | 2 | 2 (2-2) | 2% | 3 | 3 (3-0) | 1 | 7% | 1 | 1 (1-1) | 0 | 7% | |
Abbreviations: HU: Hydroxyurea; INF: Interferon alpha
Table 3A: MPN-SAF score of ET patients before and after treatment.
| Symptoms | N | Baseline | Post HU | Post HU and INF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median (IQR) | Incidence | Mean | SD | Median (IQR) | Incidence | Mean | SD | Median (IQR) | Incidence | ||
| Fatigue | 38 | 5 | 1 | 5 (4-5) | 47.4% | 4 | 1 | 4 (3-4) | 28.90% | 1 | 1 | 1 (1-2) | 10.5% |
| Early satiety | 38 | 4 | 1 | 4 (3-4) | 10.5% | 2 | 2 (2-2) | 2.60% | 1 | 0 | 1 (1-1) | 5.3% | |
| Abdominal pain | 38 | 4 | 1 | 4 (4-5) | 18.4% | 3 | 1 | 2.5 (2-3) | 10.50% | 1 | 0 | 1 (1-1) | 10.5% |
| Restlessness | 38 | 4 | 1 | 4 (4-0) | 7.9% | 3 | 3 (3-3) | 2.60% | 1 | 0 | 1 (1-1) | 5.3% | |
| Lack of concentration | 38 | 3 | 1 | 3 (3-4) | 15.8% | 3 | 3 (3-3) | 2.60% | 1 | 0 | 1 (1-1) | 5.3% | |
| Night sweating | 38 | 2 | 1 | 2 (2-3) | 39.5% | 4 | 1 | 4 (4-5) | 18.40% | 1 | 1 | 1 (1-0) | 7.9% |
| Itching | 38 | 6 | 1 | 5 (5-6) | 34.2% | 4 | 1 | 4 (3-0) | 7.90% | 1 | 0 | 1 (1-1) | 7.9% |
| Bone pain | 38 | 2 | 1 | 2 (2-3) | 36.8% | 2 | 2 (2-2) | 21.10% | 1 | 1 (1-2) | 13.2% | ||
| Fever | 38 | 3 | 1 | 3 (3-4) | 42.1% | 3 | 1 | 3 (2-3) | 28.90% | 1 | 0 | 1 (1-1) | 7.9% |
| Weight loss | 38 | 3 | 1 | 3 (3-4) | 10.5% | 3 | 3 (3-3) | 2.60% | 1 | 0 | 1 (1-1) | 5.3% | |
Abbreviations: HU: Hydroxyurea; INF: Interferon alpha
Table 3B: MPN-SAF score of PMF patients before and after treatment.
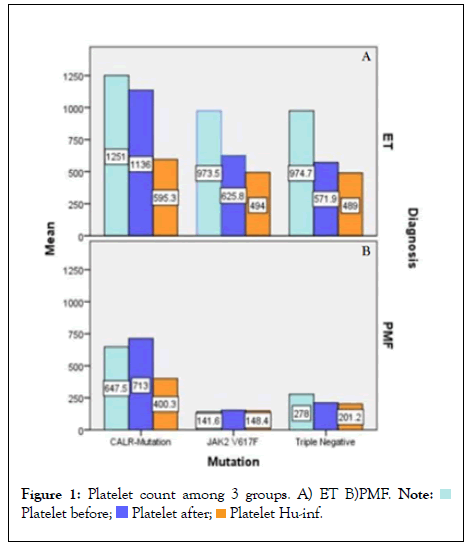
Figure 1: Platelet count among 3 groups. A) ET B)PMF. 
The management of myeloproliferative disorders in low-middle income countries has always been a challenge for clinicians because of paucity of integrated health care resources which causes unnecessary diagnostic delay and the inadequacy of treatment options consequently leading to poorer outcome of patients. The results of this study demonstrate that a significant number of JAK2 unmutated patients in our population harbored CALR mutation, which shows the significance of baseline evaluation for the driver mutations in all patients presenting with features of ET and PMF to ensure appropriate diagnosis and risk stratification. The occurrence of CALR mutation in our study correlates with previous studies published from Asian countries [16,17] however, the frequency is slightly lower to that reported in western population. Kim, et al. [18] analyzed 199 MPN cases, and reported JAK2 as the most frequently occurring abnormality (67.3%), followed by CALR (12.6%), MPL (3.5%), and triple-negative(18%) mutations. Klampfl et al, [1] initially reported a higher incidence of CALR mutations (67% in ET and 88% in PMF) in JAK2 and MPL negative patients in western population. Among the sub-types of CALR mutation, Type 1 CALR mutation was frequently found in both ET and PMF in our patients and this finding corresponds to other Asian countries except for China, where this ratio is reversed. Li N, et al. [19] has reported increase prevalence of Type 2 CALR mutation in Chinese population. One exclusive finding was the detection of homozygous CALR mutation (c.1139delA p.E380fs*50) [20], which seem to be a Type 2-like mutation in a patient with distinct clinical characteristics. This patient presented in accelerated phase of disease, massive splenomegaly and remained transfusion dependent despite of being treated with JAK2 inhibitor. Klampfl, et al. [1] previously reported three of 289 CALR-mutated patients as homozygous for the mutation, and all had type 2 mutations.
The demographic and diagnostic characteristics of CALR mutated patients in our study showed concordance with earlier studies [21,22]. Patients with CALR mutated ET and PMF demonstrated predominance of male gender, younger age, and normal to slightly low hemoglobin levels, higher platelet counts and lower thrombosis risk than those carrying the JAK2 mutation. The lower risk of thrombosis in CALR positive patients versus JAK2 positive patients has been supported by various authors, though some contradictory evidence is also found in literature [23]. This observation strengthens the view that thrombocytosis associated with CALR mutation does not predict thrombosis in MPN; rather it is the JAK2 mutation itself that triggers thrombosis (Figures 2A and 2B).
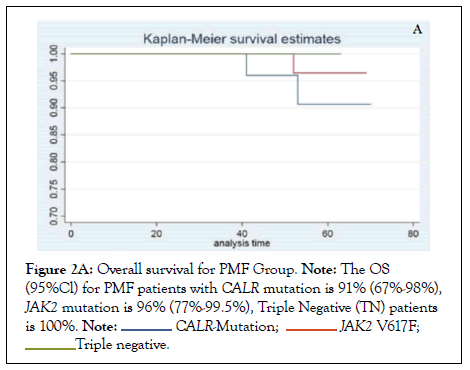
Figure 2A: Overall survival for PMF Group. Note: The OS (95%Cl) for PMF patients with CALR mutation is 91% (67%-98%), JAK2 mutation is 96% (77%-99.5%), Triple Negative (TN) patients is 100%.
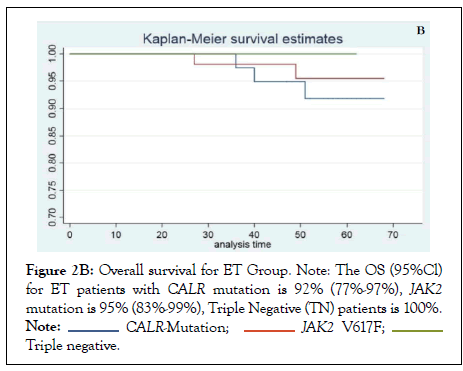
Figure 2B: Overall survival for ET Group. Note: The OS (95%Cl) for ET patients with CALR mutation is 92% (77%-97%), JAK2 mutation is 95% (83%-99%), Triple Negative (TN) patients is 100%.
In contrast to previous studies, the two CALR mutant sub-types exhibited opposing features when compared among ET and PMF. Type 2 CALR mutation was found in relatively younger age group in PMF as compared to ET whereas Type 1 CALR occurred in older age. Type 2 CALR in PMF was associated with high-risk disease as compared to Type 1 CALR in this study. No significant differences were observed in peripheral blood count or gender between the Type 1 and Type 2 mutation groups in this study. A Korean study evaluated 62 patients with ET and PMF and did not find any difference in blood counts, age, risk group and incidence of thrombosis among the two CALR sub-types [21]. There have been no controlled trials published so far comparing the differences in efficacy of standard treatment modalities in CALR versus JAK2 positive patients. In this study, despite of being in low-risk group, CALR positivity was associated with unresponsiveness to conventional first line drug in significant number of ET and PMF patients.
The prognostic value of Type 1 and Type 2 CALR mutations has been discussed in various studies. Tefferi, et al. [24] have shown that patients who carry Type 1 CALR mutation had significantly longer survival as compared to all other driver mutations. Chinese data shows association of Type 2 CALR with splenomegaly and shorter survival in these patients [16]. In contrast to that, we found splenomegaly predominantly in Type 1 CALR patients and there was no influence on survival noted in this group. Leukemic transformation occurred equally in Type 1 and Type 2 CALR mutated patients with PMF in this study. There was no statistically significant impact of CALR mutation and its sub-types on overall survival and prognosis of patients as compared to JAK2 mutated patients with ET and PMF. Small number of patients in each subgroup may be attributable to the inconsistency observed in our study.
CALR mutation comprised of 32.4% of ET and 38.1% of PMF with non-mutated JAK2 in our study group, therefore we strongly suggest performing CALR mutation analysis for the diagnosis of JAK2 negative MPN in our population. Though, CALR mutation did not produce significant impact on overall survival and prognosis when compared with JAK2 mutation, it poses a robust clinical challenge because of refractoriness and resistance in substantial number of patients, to the conventional treatment modalities available in Pakistan. Long-term follow up is required to determine the clinical relevance and response to treatment in CALR mutated ET and PMF.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Uzma Zaidi] and [Maham Fayyaz & Muhammad Nizamuddin] respectively. The first draft of the manuscript was written by [Uzma Zaidi]. All authors read and approved the final manuscript.
Acknowledgments of people, grants, funds, etc. should be placed in a separate section on the title.
We did not receive any financial assistance for this study.
All authors declared that they have no conflicts of interest.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the National Institute of Blood Diseases & Bone Marrow Transplantation and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee of NIBD & BMT (NIBD/RD-IRB#141/10-2014).
Informed voluntarily consent was obtained from all patients.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
[CrossRef], [Google Scholar], [PubMed]
Citation: Zaidi U, Rashid M, Ahmed RZ, Fayyaz M, Nizamuddin M, Anwar N, et al. (2022) Management of Calreticulin Mutated Myeloproliferative Neoplasms: Real-world Outcomes from a Low-Middle Income Country. J Leuk. 10:309.
Received: 05-Aug-2022, Manuscript No. JLU-22-18720; Editor assigned: 10-Aug-2022, Pre QC No. JLU-22-18720 (PQ); Reviewed: 26-Aug-2022, QC No. JLU-22-18720; Revised: 02-Sep-2022, Manuscript No. JLU-22-18720 (R); Published: 12-Sep-2022 , DOI: 10.35248/2329-6917.22.10.309
Copyright: © 2022 Zaidi U, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.