Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2015) Volume 6, Issue 3
A catalyst of manganese oxides (MnOx) supported on fly ash-palygorskite (MnOx/FA-PG) was prepared for low temperature selective catalytic reduction (SCR) of nitric oxides (NOx) by ammonia (NH3). The influences of the preparation method, active species precursors, calcination temperature, calcination time and particle size on the SCR performance of the MnOx/FA-PG catalyst were studied. Catalysts were characterized by Brunauer-Emmett-Teller (BET) analysis, scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray fluorescent spectrometry (XRF), and NH3 adsorption and temperature programmed desorption (TPD) to illustrate the physic-chemical properties of catalysts. Results showed that a negligible difference in SCR activity is observed on the MnOx/FA-PG catalyst prepared by heterogeneous deposition and wetness impregnation. Activity of the MnOx/FA-PG catalyst prepared by wetness impregnation with Mn(NO3)2 as precursor was significantly superior to that of prepared with Mn(CH3COO)2 as precursor. Moreover, calcination temperature significantly influenced catalytic activity. Over 70% NO conversion could be achieved at 100°C for Mn8/FA-PG catalysts calcined at 400°C for 3 h. The active component of the catalysts mainly existed in Mn4+. According to the results of NH3 adsorption and temperature programmed desorption (TPD), adsorption capacity of NH3 was not the decisive factor of catalytic activity and the key was the activation of adsorbed NH3.
Keywords: Fly ash-palygorskite; Manganese oxides; Lowtemperature SCR
Over the past decades, nitrogen oxides from power plants and motor vehicles have been remained as major air pollutants. They can result in acid rain, photochemical smog, and ozone depletion [1,2]. Selective catalytic reduction (SCR) of NOx with NH3 in the presence of oxygen is a proven technique for the removal of NOx from flue gases [3]. A large number of SCR catalysts have been explored, including noble metals [4,5], transition metal oxides [6,7] and zeolites [8,9]. Especially, V2O5-WO3/TiO2 based catalysts have been extensively used in the industry [10,11]. However, the operating temperature for these catalysts is usually above 300°C, and the exhaust gases usually contain a large number of fly ash and SO2, which can easily deactivate the catalysts. Thus, the development of active SCR catalysts that can be operated at relatively low temperatures is needed.
At present, many transition metal oxides are extensively studied and proved to be highly active for low temperature SCR of NO with NH3, among which MnOx-supported catalysts attract the most concern [10,12-14]. Zhao et al. found that Mn0.06/Al2O3 yields the highest NO conversion and 80% of NO conversion is obtained at 250°C and 15,000 h-1 [15]. Zhang et al. reported that Mn/TiO2 catalysts exhibit a high NO conversion of 90% at 100°C [16]. The high SCR activity for MnOx- supported catalysts implies that there may be an intimate connection between manganese oxides and carrier. Smirniotis et al. compared TiO2-, Al2O3- and SiO2-supported MnOx catalysts and found that the SCR performance of the supported Mn catalysts decreased in the following order: TiO2 (anatase, high surface area), TiO2 (rutile), TiO2 (anatase, rutile), γ-Al2O3, SiO2, TiO2 (anatase, low surface area) [17]. The material of carrier is directly related to the SCR activity for manganese-based catalysts.
In this paper, a solid waste material, fly ash (FA), was used as support, which can cause many problems and has become an important environmental issue [18]. Due to its moderate pore volume and pore structure, as well as its high silica and alumina contents, FA is used to prepare zeolite materials. Besides, a clay mineral, palygorskite (PG) [19,20], was added as a binder to FA. The materials were mixed well after machine extrusion to provide an inexpensive catalyst support [21]. Manganese oxide (MnOx) was loaded as the active ingredient to produce a MnOx -supported catalyst (MnOx/FA-PG). This catalyst was then characterized through experiments in a fixed bed reactor to investigate the feasibility of using FA-PG as a catalyst support. Specific surface area [Brunauer-Emmett-Teller (BET)] analysis, scanning electron microscopy (SEM), X-ray diffraction (XRD) and X-ray fluorescent spectrometry (XRF) were used to characterise the surface properties and crystalline structures of the catalyst. NH3 adsorption and temperature programmed desorption (TPD) were performed to evaluate the effect of manganese species on the surface acidity of the catalyst.
Catalyst preparation
FA was obtained from the Anqing Power Plant in Anhui Province, and natural PG was acquired from Jiashan County. Main chemical composition of the molded fly ash and palygorskite are listed in Table 1. Four particle size ranges, i.e., 5-8 mm, 2-5 mm, 0.38-0.83 mm and 0.25-0.38 mm, were chosen as the experimental samples. FA-PG was pretreated at 300°C for 3 h to remove surface water. The Mnx/ FA-PG catalysts were prepared firstly by heterogeneous precipitation and wetness impregnation of heat-pretreated FA-PG with Mn nitrate aqueous solution. The concentration of the solution was varied to yield different Mn loading contents. The samples were dried overnight at 110°C. Then, the specific particle sizes of catalysts were calcined in O2 stream at different temperatures and times. The catalyst samples were labeled Mnx/FA-PG, MA-Mnx/FA-PG and Mnx(P)/FA-PG based on loading of the active component with different precursors and preparation methods (“x”, “MA” and “P” refer to the weight percentage of Mn loading, the Mn(CH3COO)2 solution and the heterogeneous precipitation method, respectively).
| Sample | SiO2 | AlO3 | Fe2O3 | CaO | MgO | TiO2 | K2O | MnO | P2O5 | SO3 | FeO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | 48.29 | 21.64 | 3.05 | 20.33 | 1.74 | 0.98 | 1.66 | 0.04 | 0.26 | 1.87 | / |
| PG | 69.44 | 11.84 | 5.139 | 0.211 | 12.16 | 0.43 | 0.5 | 0.046 | 0.03 | / | 0.08 |
Table 1: Main chemical composition of FA and PG (%).
Catalyst characterization
X-ray fluorescent spectrometry (XRF) was performed on a XRF-1800 analyses (Shimadzu, Japan) to measure the chemical composition of the catalyst. X-ray Diffraction (XRD) was performed on a D/MAx2500V diffractometer (Rigaku, Japan) equipped with a monochromated Cu Kα radiation source. The catalysts were scanned at 2θ ranging from 10°-70°. Scanning electron microscopy (SEM) images of the samples were obtained by a JSM-6490LV (Hitachi, Japan) analyser.
Brunauer-Emmett-Teller (BET) surface area was measured from the N2 adsorption and desorption isotherms at -196°C using the NOVA 2200e (Quantachrome, American) adsorption analyser. Prior to analysis, 0.08-0.1 g of catalyst was preheated at 100°C for 2 h under a N2 atmosphere. NH3 adsorption and temperature programmed desorption (TPD) were conducted in a quartz microreactor. Then, 100 mg of the sample was preheated in Ar at 473 K for 1 h to remove any adsorbed species. Afterward, the sample was cooled to 323 K in Ar (100 mL·min-1) and 1500 ppm NH3 balanced by Ar was adsorbed at this temperature with a total flow rate of 100 mL·min-1 until no signal variation of NH3 was detected. Subsequently, the sample was purged with 100 mL·min-1 Ar until no NH3 was detected in the outlet, and then ramped to 1073 K at a linear heating rate of 10 K·min-1 in Ar (100 mL·min-1). The analysis of gases was performed using an online mass spectrometer QIC2000 (Hidden, Germany).
Activity measurement
Catalyst activity was evaluated in a quartz fixed bed continuous flow reactor that operated at a pressure of 0.1 MP, and 3 g of sample was used in each test. Gas composition was 600 ppm NO, 600 ppm NH3 and 3 vol% O2 balanced by Ar. Total flow rate was 350 mL·min-1, which corresponds to a gas hour space velocity of 4000 h-1. NO concentrations in the inlet and outlet gases were measured online with a flue gas analyser (testo350-XL). Catalyst performance was estimated through NO conversion, which was calculated by the following equation:
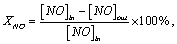 (1)
(1)
Where 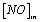 and
and 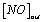 denote the inlet and outlet concentrations of NO, respectively.
denote the inlet and outlet concentrations of NO, respectively.
Effect of different precursors and loadings on SCR activity over catalysts
NO conversion of Mnx/FA-PG catalyst prepared using Mn(NO3)2 as a precursor is shown in Figure 1. NO conversion increased with temperature increasing at the temperature range of 100-250°C with Mn-loading. Then, it started to decrease at temperature higher than 250°C. Besides, at the same temperature, NO removal efficiency increased with increasing Mn-loading. NO conversion for Mn24/ FA-PG catalyst could reach to 100% at 100°C. For FA-PG, the NO conversion was much lower in a temperature range of 100-200°C, indicating that the low temperature SCR performance of Mn-loaded catalyst was not contributed by the carrier but the MnOx itself. As shown in Figure 2, NO conversion for the MA-Mnx/FA-PG catalyst prepared using Mn(CH3COO)2 as precursor was higher than that of FA-PG over the entire reaction temperature, but significantly lower than that of the Mnx/FA-PG catalyst under the same Mn-loading. It is reasonable deduced that the difference of the precursors may form different active species after calcination. Researchers also investigated the characteristics of the catalysts prepared by different precursors. However, no consensus was reached. Li et al. [22] reported that the catalyst prepared using Mn(NO3)2 as precursor after calcination at 400°C mainly formed MnO2 species. Meanwhile, Mn2O3 species was formed when Mn(CH3COO)2 was used as precursor. In addition, the catalytic activity of Mn2O3 was higher than that of MnO2 in the unit area. However, Wu et al. [23] considered that the catalytic activity of MnOx could be sequenced as follows: MnO2>Mn5O8>Mn2O3>Mn3O4>MnO. Further explanations about the characteristics of the catalysts prepared by different precursors will illustrate in the following.
Effect of preparation method on SCR activity
From above results, catalysts prepared by Mn(NO3)2 as precursor had high SCR activity. Thus, the NO conversion of the Mn8/FAPG/ 300°C and Mn8(P)/FA-PG/300°C catalysts prepared by Mn(NO3)2 as precursor at the entire reaction temperature range is shown in Figure 3. As shown in Figure 3, Mn8(P)/FA-PG catalyst had higher NO conversion than Mn8/FA-PG catalyst in the temperature range of 100- 150°C. However, at the temperature higher than 200°C, NO conversion of Mn8(P)/FA-PG was lower than that of the Mn8/FA-PG catalyst. Besides, the preparation process for impregnation method was complex and led to the loss of raw materials during catalyst preparation. Based on the aforementioned results, the impregnation method is optimal and relatively economic.
Effect of calcination temperature and calcination time on SCR activity
Based on the preceding catalyst characterization, calcination temperature and calcination time have a significant effect on the characteristics of the catalysts. In this work, we also study the effect of calcination temperature and calcination time on SCR activity. As shown in Figure 4a, the NO conversion of the Mnx/FA-PG catalyst first gradually increased, and then decreased slightly with increasing calcination temperature. When calcination temperature was 400°C, the highest SCR activity was obtained at a low temperature. In particular, over 70% NO conversion was got at 100°C and approximately 100% at 150°C. Hence, the Mnx/FA-PG catalyst that calcined at 400°C had higher SCR activity. A low calcination temperature may lead to the incomplete decomposition of Mn(NO3)2 which is accompanied with NOx production during the reaction process. However, at a high calcination temperature, the structure of gesso-water and structure-water in PG might fold, which can lead to the collapse of passageways in FA-PG carriers; this condition is disadvantageous for NH3 adsorption [24]. The surface areas of Mnx/FA-PG catalysts under different calcination temperatures are listed in Table 2. Compared with the carrier of FA-PG, the catalysts calcined at 300°C and 400°C had large BET surface areas, but the difference between them was minimal. Meanwhile, for the catalyst calcined at 500°C, the BET surface area decreased to a certain extent. A conclusion could be drawn that an extremely high calcination temperature leads to the destruction of the support structural. Besides, the NO conversion of the Mn8/FA-PG catalyst calcined at 400°C for different times is shown in Figure 4b. As shown in Figure 4b, the NO conversion of the Mnx/FA-PG catalyst gradually decreased with increasing calcination times. Highest SCR activity was obtained when calcined at 400°C for 3 h. Thus, catalyst of Mn8/FA-PG mentioned below was all calcined at 400°C for 3 h.
| Sample | Parameter | SBET [m2/g] |
|---|---|---|
| FA-PG | 0.38-0.83mm | 37.20 |
| Mn8/FA-PGa | Impregnation | 68.70 |
| Mn8(P)/FA-PGb | Precipitation | 72.10 |
| Mn8/FA-PG | Mn(NO3)2 | 68.70 |
| MA-Mn8/FA-PGc | Mn(CH3COO)2 | 44.04 |
| Mn8/FA-PG/300°Cd | 300°C | 56.28 |
| Mn8/FA-PG/400°Ce | 400°C | 49.05 |
| Mn8/FA-PG/500°Cf | 500°C | 29.22 |
| Mn8/FA-PG | 4.00-8.00 mm | 63.32 |
| Mn8/FA-PG | 2.00-4.00 mm | 65.66 |
| Mn8/FA-PG | 0.38-0.83 mm | 68.70 |
| Mn8/FA-PG | 0.25-0.38 mm | 64.53 |
SBET: Specific surface area
Table 2: BET surface area of catalysts. (a) Mn8/FA-PG was prepared by impregnation method with Mn(NO3) as precursor. (b) Mn8(P)/FA-PG was prepared via the heterogeneous precipitation method with Mn(NO3)2 as precursor. (c) MA-Mnx/ FA-PG prepared using Mn(CH3COO)2 as precursor. (d) Mn8/FA-PG/300°C calcined at 300°C. (e) Mn8/FA-PG/400°C calcined at 400°C. (f) Mn8/FA-PG/ 500°C calcined at 500°C.
Effect of particle size on SCR activity over catalysts
Catalyst particle size has a significant effect on SCR activity. In the preparation method described above, the Mn8/FA-PG (4.00-8.00 mm), Mn8/FA-PG (2.00-4.00 mm), Mn8/FA-PG (0.38-0.83 mm) and Mn8/FA-PG (0.25-0.38 mm) catalysts were prepared to evaluate the influence of particle size on SCR activity for Mn-based catalysts. As illustrated in Figure 5, the NO conversion of the Mn8/FA-PG catalyst first gradually increased, and then decreased slightly with decreasing particle size. The Mn8/FA-PG catalyst with a particle size of 0.38-0.83 mm had a highest SCR activity, which is different from the results of Xuan [25,26]. The difference can be attributed to the material of the carrier and the preparation methods. The specific surface areas of Mn8/ FA-PG catalysts under different situations are listed in Table 2. Mn8/ FA-PG catalyst with a particle size of 0.38-0.83 mm had largest surface area. According to Eley-Ridal mechanism [27], the catalyst with large surface area is more conductive to the reaction gases (NH3 and NOx) adsorbed on the carrier leading to the enhancement of SCR activity.
XRD analyses
The XRD patterns of the Mnx/FA-PG catalyst prepared by wetness impregnation with Mn(NO3)2 and Mn(CH3COO)2 as precursors are shown in Figure 6. As shown in Figure 6a, all these patterns presented intense peaks of PG, kaolinite and dolomite due to the complex composition of FA-PG. However, there were no diffraction peaks of manganese oxides for Mn8/FA-PG and MA-Mn8/FA-PG catalysts calcined at 400°C indicating that the complex composition of FAPG may influence the diffraction of MnOx or MnOx is uniformly dispersed on the surface of the carrier with a low percentage of active components. Thus, in this study, we particularly selected the Mn12/ FA-PG and MA-Mn12/FA-PG catalysts for XRD analyses to illustrate the presence of manganese oxides. As shown in Figures 6d and 6e, the diffraction peaks of crystalline MnO2 and Mn2O3 phases were observed in the Mn12/FA-PG and MA-Mn12/FA-PG catalysts respectively, suggesting that MnOx in the amorphous phase is uniformly dispersed on the surface of the carrier, which is consistent with the founding by Li [22]. This finding indicated that MnOx was mainly in the form of MnO2 when the Mnx/FA-PG catalyst was calcined at 400°C with Mn(NO3)2 as precursor. Meanwhile, the Mn(CH3COO)2 precursor mainly formed Mn2O3 species. Based on the above results in Figures 1 and 2, it is reasonable deduced that catalytic activity of MnO2>Mn2O3 in the unit area. The XRD patterns of the Mn12/FA-PG catalyst calcined at different temperatures are shown in Figure 7. The intensity of the diffraction peak of PG, kaolinite and dolomite for Mn-based catalyst decreased with increasing calcination temperature indicating that a high calcination temperature may destroy the primary structure of the carrier. Besides, the peaks corresponding to crystalline MnO2 phase diminished remarkably along with increasing calcination temperature and disappeared completely when calcination temperature reached over 400°C. When calcination temperature was 500°C, the diffraction peaks of Mn2O3 appeared. Moreover, the diffraction peaks of Mn2O3 were most prominent when calcination temperature reached to 600°C. This phenomenon is accordance with the founding by Kapteijn [28] that different calcination temperatures result in various oxidation states of manganese oxides and a high calcination temperature leads to a low valence of Mn.
SEM of the catalysts
The electron microscopy images of the Mnx/FA-PG catalyst prepared via different methods are shown in Figure 8. These images (b-d) pertaining to the Mn8/FA-PG catalyst prepared by wetness impregnation with Mn(NO3)2, Mn(CH3COO)2 as precursors and heterogeneous precipitation method, respectively. Compared to the carrier of FA-PG, there was a certain amount of white particles existed for all of the Mn-loaded catalysts, revealing that MnOx has dispersed on the surface of the catalyst after calcination. However, for Mn8/FAPG catalyst prepared with Mn(NO3)2 as precursor, the particles on the surface of the catalyst were obviously more and higher uniformly dispersed than that of MA-Mn8/FA-PG catalyst prepared with Mn(CH3COO)2, indicating that the precursor remarkably influences the morphologies of catalysts. Moreover, for MA-Mn8/FA-PG catalyst, there was a slight sintering phenomenon after being calcined, which is not conducive to improving catalyst activity. The SEM images of Mn8/FA-PG and MA-Mn8/FA-PG were agreed with the activity test in Figures 1 and 2. Besides, as shown in Figure 8d, most white globular substances (MnOx) appeared on the surface of Mn8/FA-PG catalyst prepared by heterogeneous deposition method illustrated that the catalyst exhibited the best SCR activity compared to the others [29].
BET analyses
The physical properties of catalysts are important in determining the adsorption-desorption phenomena of gases onto its surface and influencing the activity of catalysts. Catalyst with large surface area is more conductive to the reaction gases (NH3 and NOx) adsorbed on the carrier leading to the enhancement of SCR activity. Thus, the BET analyses are given and the textural properties are summarized in Table 2. Compared to the carrier of FA-PG, the surface area of catalysts increased after Mn-loaded. Mn8(P)/FA-PG prepared by heterogeneous precipitation had larger surface area than Mn8/FA-PG with impregnation. Combined with activity tests in Figure 3, it can be concluded that the larger surface area accounts for the improvement of SCR activity for Mn8(P)/FA-PG. Besides, for Mn8/FA-PG prepared with Mn(NO3)2 as precursor, the BET surface area was higher than that of MA-Mn8/FA-PG prepared with Mn(CH3COO)2 as precursor. Similar result was obtained for Mn8/FA-PG prepared with Mn(NO3)2 as precursor. For Mnx/FA-PG catalyst with different size of particles, the catalyst with a particle size of 0.38 mm to 0.83 mm has largest surface area corresponding to the SCR activity tests in Figure 5. The specific surface areas of the Mnx/FA-PG catalyst calcined at 300°C and 400°C both exhibited a significant increase compare to the FAPG carrier. However, the NO conversion of the Mnx/FA-PG decreased when calcined at 500°C because the carrier structure was destroyed when calcination temperature was too high.
NH3 adsorption analyses
A comparative study of NH3 adsorption for the Mn(x)/FA-PG catalyst with different Mn loadings is presented in Figure 9. The penetration time of NH3 was observed to decrease as Mn loading increased. This finding showed that the FA-PG carrier has a higher adsorption capacity than others. However, according to the results of BET analyses and activity test in Figure 1, the surface area of catalysts increased and the activity was improved after Mn-loaded. Thus, it can be deduced that the surface area of catalysts is not the decisive factor for NH3 adsorption and the adsorption capacity of NH3 not directly influence the SCR activity. Busca et al. [11] determined that the adsorption capacity of NH3 influences catalytic activity. However, it was not the decisive factor and the key was the activation of adsorbed NH3.
NH3-TPD analyses of catalysts
NH3-TPD profiles of different Mnx/FA-PG catalysts are shown in Figure 10. Three main desorption peaks with different strength were observed at 132°C, 390°C and 603°C for FA-PG in the entire temperature range indicating that the carrier of FA-PG has three obvious acid sites. However, after manganese oxides loaded on the FAPG, only one desorption peak at 132°C was found, and the desorption peak at 390°C and 603°C were disappeared. The peak at 132°C for Mnx/ FA-PG could be assigned to the physically adsorbed of NH3. According to the founding by Greenhalgh [30], the strength of B-acid site gradually decreased with the exchange of metal ion due to the reaction between metal ions of the precursors and OH on the support surface. Therefore, the peak at 390 oC and 603 oC may be caused by desorption of NH3 that was adsorbed on the B-acid site of the carrier itself. Besides, Busca et al. [11] indicated that, the SCR performance of Mn-based catalyst is not related to the adsorption and activation of the B-acid site, but the L-acid site.
Mnx/FA-PG catalyst was highly active for SCR of NO with NH3 at a temperature range of 150-300°C. Catalysts prepared by impregnation with Mn(NO3)2 as precursor not only efficient but also relatively economic owning to the uniformly dispersed and high catalytic activity of MnOx on the surface of the catalyst. NO conversion closed to 100% at 100°C under Mn-loading of 24% with particle size of 0.38 mm to 0.83 mm. Moreover, catalysts calcined at 400°C for 3 h exhibited the highest activity. Most important, the SCR performance of Mn-based catalyst was not related to the adsorption and activation of the B-acid site, but the L-acid site.
The authors gratefully acknowledge the financial support received from the National Natural Science Foundation of China (Grants Nos. 40902020, 51002042) and the Ph.D. Programmes Foundation of the Ministry of Education of China (Grant No. 20090111120019).