Angiology: Open Access
Open Access
ISSN: 2329-9495
ISSN: 2329-9495
Research Article - (2020)Volume 8, Issue 1
Background: Short-term intensive training programs result in a significant improvement in the walking ability of most patients with stable peripheral artery disease (PAD). This single-center study is aimed at comparing outcome parameters of PAD patients with intermittent claudication undergoing a long-term physiotherapist-delivered walking exercise program (WEP) with those of a usual-care PAD therapy.
Patients and Methods: Using a matched-pair analysis, the data of symptomatic PAD patients who joined a long-term home-based WEP on a community level was compared to those of an appropriate usual-care PAD patient control cohort without regular exercise therapy. The predictor variable was WEP (WEP participation compared with usualcare PAD therapy) and the main outcome variable was the number of endovascular or surgical procedures over time. Results: Over a period of 25 years (January 1, 1990 to December 31, 2015), 309 WEP participants (164, 53.1% males) took part for a mean of 53.9 months (range 3 to 251). Adapted for 10 patient years there was an identical frequency of PAD-related limb events or other cardiovascular events in both cohorts, but in the WEP group significantly fewer revascularization procedures were undertaken than in corresponding controls (HR 1.34, 95% CI 1.22-1.75 vs. HR 1.58, 95% CI 1.44-1.75; p<0.001). The mean survival time of WEP patients was 165.5 months (median 169.2 SE 5.01) and that of usual-care patients 135.8 months (median 116.1 SE 5.53).
Conclusions: Participation in a long-term home-based WEP is associated with lower demand for endovascular or surgical therapies and an extended lifespan. It is unclear whether this is the result of program participation or sequelae of a generally modified life-style of WEP participants. Prospective randomized studies are required that establish the efficacy of a structured walking exercise program in the community alongside usual care therapy.
Peripheral arterial disease; Long-Term physical exercise; Intermittent claudication; Secondary prevention
HEPs: Home-Based Exercise Programs; PAD: Peripheral Artery Disease; PS: Propensity Score; SET: Supervised Exercise Training; WEP: Walking Exercise Program
Walking exercise training is an effective measure for improving the ambulatory dysfunction of patients with peripheral artery disease (PAD). Many studies have proven the benefit of short-term intensive exercise training for symptomatic PAD patients, but the results mainly originate from selected rehabilitation program participants or relatively small clinical cohorts [1,2]. Considering the chronically progressive course of cardiovascular diseases, it is unclear whether the positive effects on walking ability achieved are of a long-lasting nature. As known from cardiac rehabilitation studies, regular long-term physical activity is required to achieve ongoing effective secondary prevention and health promotion benefits in both middle-aged and elderly patients with coronary artery disease [3]. In Germany, PAD patients with intermittent claudication have the opportunity to attend a long-term rehabilitation program in a community-based setting. Health insurance funds reimburse the expenses for this program, provided it is prescribed by a doctor and supervised by a physiotherapist. However, the willingness of patients to participate in long-term programs for the secondary prevention of cardiovascular diseases is generally low, deficiencies in uptake and adherence of PAD patients are evident [4-6]. The objective of this single-center retrospective open cohort study was to examine whether participation in a long-term walking exercise program (WEP) has an impact on the need for endovascular or surgical treatment in PAD patients compared with usual care.
Study setting
In the city of Karlsruhe, located in the south-west region of Germany, the first regional walking exercise groups were founded in 1990 and have been continually working up to now. Under the supervision of physiotherapists, the training sessions are undertaken twice a week in twelve training centers spread over the city. The patients are assigned to the training program by primary care doctors or specialists. Precise data of individual WEP program adherence is available because the patient's signature for every completed training session is required for billing by the insurance provider.
Study population
Over a period of 25 years (January 1, 1990, to December 31, 2015) willing patients entered the program on a rolling admission policy, resulting in follow-up periods of variable length. All patients whose signatures were recorded at least three consecutive times in the electronic database of supervisory exercise training physiotherapists were enrolled in the study. The duration of participation was defined as the period between the first and last signature. A corresponding usual-care patient was chosen for each WEP patient using matched-pair analysis. Key variables (e.g. age, sex) and cardiovascular risk factors as confounding variables were selected for the matching procedure. All patients, regardless of their assignment to the WEP group or control group, received usual care as prescribed by the national PAD guidelines, including lifestyle modification, risk factor management, and pharmacotherapy. The institutional review board at the University of Heidelberg approved the project (approval registration no. S-446), and the study complied fully with the recommendations of the Declaration of Helsinki.
Exclusion criteria
All patients were diagnosed with PAD using Doppler pressure measurement of extremity arteries, color duplex sonography and constant-load treadmill test (10% slope, 3 km/h). Only PAD patients with intermittent claudication (Fontaine classification stage II) were selected for participation in the WEP program. Exclusion criteria were unstable angina, severe heart failure (NYHA class III or higher), inability to walk as a result of major amputation, advanced neurological disorders or severe osteoarthrosis.
Walking exercise program
Every session is delivered by trained, registered physiotherapists. After a 10-minute warming-up period doing diverse gymnastics, participants undertake a first walking training period of about 10 minutes, containing episodes of forced walking and different gait patterns (e.g., heel-to-toe walk). Interrupted by leg exercises while seated and accompanied by rhythmic music, walking episodes are repeated twice. The session concludes with a low-intensity common ball game. Finally, each participant is provided with a pedometer and walks until claudication pain occurs. The maximum number of painless steps is recorded. The homework assignment includes daily toe walk and various walking exercises.
The patient characteristics and comorbidities between PAD patients and control patients were compared using student's t test, Mann- Whitney-U test and Fisher-Exact test, respectively. All statistical procedures were performed with SPSS 15.0 software (SPSS Inc., Chicago, Ill.). Matching was performed with the Propensity Score (PS) method proposed by Rosenbaum and Rubin [7]. Binary logistic regression determined the patient's age, gender and comorbidities as significant determinants for PAD. Corresponding regression coefficients were used to calculate the PS for matching. Survival times were presented with a Kaplan-Meier estimator and compared with a Log Rank test.
A total of 338 subject signatures were registered in the exercise training database. Twenty-one persons (6.2%) participated less than three times and did not fulfill the inclusion criteria. Eight patients (2.3%) dropped out because no information on stay and outcome could be obtained. Hence, the WEP group comprised 309 patients which could be included in the matched-pair analysis.
Baseline clinical characteristics
The patient baseline characteristics of the WEP group and matchedpair control group are shown in Table 1. Distribution of patient characteristics and cardiovascular risk factors basically correspond in both groups.
| Exercise group (n=309) | Control group (n=309) | p value | |
|---|---|---|---|
| Age, mean (SD)* | 69.3 (8.1) | 69.1 (7.9) | 0.220 |
| Sex Males, n (%) Females, n (%) |
164 (53.1) 145 (46.9) |
164 (53.1) 145 (46.9) |
|
| Body mass index (kg/m2) Males (± SD) Females (± SD) |
26.8 ± 3.8 26.4 ± 4.1 |
27.5 ± 4.2 25.5 ± 4.9 |
0.197 0.218 |
| Hypertension, n (%) | 207 (67) | 238 (77) | 0.007 |
| Diabetes, n (%) | 84 (27.2) | 92 (29.8) | 0.533 |
| Dyslipidemia, n (%) | 103 (33.3) | 91 (29.4) | 0.340 |
| Smoking Current smoking, n (%) Pack-years (± SD) |
119 (38.6) 32.3 ± 24.6 |
134 (43.5) 46.4 ±25.2 |
0.252 <0.001 |
| Ankle-brachial index (± SD) | 0.62 ± 0.17 | 0.60 ± 0.15 | 0.223 |
*Year listed according to matched-pair analysis
Table 1: Baseline patient characteristics.
Walking exercise program attendance and adherence
The follow-up period of PAD patients participating in exercise training and usual-care controls was calculated as the difference between the first consultation and the end of the study on 31.12.2015 or the date of death if the patient died beforehand. Patients participated in the WEP program on average for four and a half years (53.9 months). Deducting the months missing a signature within the entire documented participation period, there was a 90% adherence of participants to the WEP program. As a consequence, each participant completed 320 sessions on average.
PAD parameters at baseline and follow-up
Baseline measurements of the total patient study sample (n=535; missing values due to medial calcific sclerosis or edema) indicated an initial correlation between ABI and treadmill walking distance (pain-free walking distance, rs 0.182; p<0.001, maximum walking distance, rs 0.238; p<0.001). The walking distance of WEP patients measured by pedometer correlated significantly at baseline, after 5 years and after 10 years with the walking distance on the treadmill as well as with initially measured ABI values Table 2. To assess the influence of ABI at baseline on survival time, a Cox regression was calculated showing a significant correlation between ABI and patient's survival time (p<0.001). The associated hazard ratio was 0.314 (95% CI 0.160-0.614).
| Variable | n* | rs** | p value | |
|---|---|---|---|---|
| Baseline | 280 | 0.236 | <0.001 | |
| Treadmill pain-free walking distance |
5 years | 89 | 0.297 | 0.005 |
| 10 years | 25 | 0.442 | 0.027 | |
| Baseline | 280 | 0.324 | <0.001 | |
| Treadmill maximum walking distance |
5 years | 89 | 0.267 | 0.012 |
| 10 years | 25 | 0.459 | 0.021 | |
| Baseline | 301 | 0.181 | 0.002 | |
| Ankle-brachial index | 5 years | 90 | 0.141 | 0.185 |
| 10 years | 29 | 0.178 | 0.356 |
* Values not available in all records e.g. due to medial calcific sclerosis or edema
**Spearman’s rank correlation coefficient
Table 2: Correlation between pedometer walking distance, treadmill walking distance and ankle-brachial index
Figure 1 shows that the walking distance of WEP patients measured on the pedometer during the initial 36 months of participation increased and subsequently declined within the following 17 months. Only 55 months are depicted in the figure because the evaluable number of participants subsequently amounted to only one third.
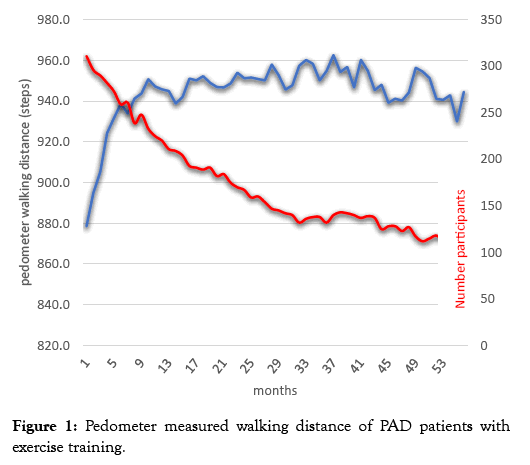
Figure 1: Pedometer measured walking distance of PAD patients with exercise training.
The number of cardiovascular events and endovascular revascularizations were statistically related to the respective followup period (Poisson distribution). Adapted for 10 patient years, the WEP participants had fewer PAD procedures (hazard ratio 1.34, 95% CI 1.22-1.75 vs. hazard ratio 1.58, 95% CI 1.44-1.75; p<0.001) than corresponding controls Table 3.
| Exercise group (n=309) | Control group (n=309) | p vaue | |||
|---|---|---|---|---|---|
| Total number | Number per 10 patient-years |
Total number | Number per 10 patient-years |
||
| PAD events§ | 33 | 0.10 (0.07; 0.14) |
37 | 0.14 (0.10; 0.19) |
0.633 |
| Sum of CAD and carotid events§§ | 77 | 0.23 (0.18; 0.28) |
63 | 0.24 (0.18; 0.31) |
0.490 |
| PAD procedures* | 101 | 1.34 (1.22;1.75) |
175 | 1.58 (1.44; 1.75) |
< 0.001 |
| Sum of CAD and carotid procedures** | 81 | 0.24 (0.19; 0.30) |
91 | 0.35 (0.28; 0.43) |
0.446 |
PAD: Peripheral Arterial Disease; CAD: Coronary Artery Disease; data depicts mean number (95% confidence interval)
§ Limb gangrene or ulceration
§§ Myocardial infarction, stroke
* PAD stent/operation
** Coronary stent/operation, carotid stent/operation
Table 3: Frequency of cardiovascular events and endovascular therapies in PAD patients participating in exercise training and usual-care controls.
There was a significant difference between the mean survival time of WEP patients (165.5 months, median 169.2 SE 5.01) and that of usual care patients (135.8 months, median 116.1 SE 5.53) (Log Rank test <0.001) Figure 2.
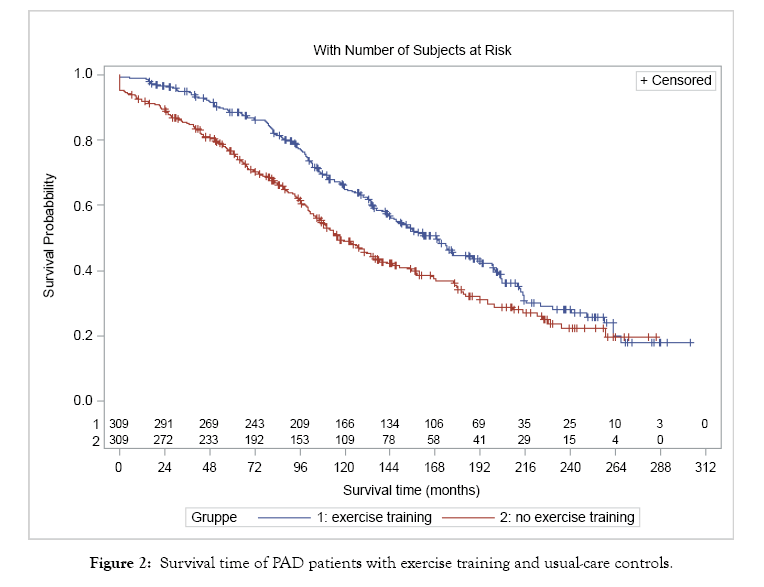
Figure 2: Survival time of PAD patients with exercise training and usual-care controls.
The findings of our study show that WEP-participating PAD patients initially improved their pedometer-measured walking distance and maintained it at a consistently high level over the following period. Structured home-based exercise programs (HEPs)–and we would classify our community-based walking exercise program as one of the above – are considered as therapeutic alternatives if supervised exercise training (SET) is unavailable or not feasible [8,9]. However, strong evidence supporting HEP in comparison to usual care is still lacking [10,11]. A systematic review by Al-Jundi et al. suggested “low-level” evidence supporting the efficacy of HEPs [11]. Equally, a recent meta-analysis showed that structured home-based exercise programs are effective at improving walking endurance and physical activity in the short term for patients with PAD [12]. The development of walking performance in our longterm study is in line with Menard et al. [13] who followed up 63 PAD patients (mean follow-up period=48 months) who had experienced improvement in claudication distance after completing a 12-week supervised intensive exercise training program. Only those patients who continued to exercise a minimum of 60 min/week maintained increased claudication performance, whereas the walking values returned to baseline for the sedentary group. Regarding the retrospective design of our study with a rapidly decreasing number of participants, discontinuous treadmill measurements and increasing age-related fragility of the patients, a more detailed analysis of treadmill-measured walking capacity was abandoned. Interestingly, recent insights indicate that the determination of the treadmill-measured walking capacity of PAD patients does not exactly correlate with their daily physical activity validated on a pedometer [14,15]. As a result, Gommans et al. recommend an assessment of physical activity as an additional outcome measure for the care of claudicating patients and its evaluation in PAD studies [14].
Generally, the rationale for recommending walking exercise for symptomatic PAD patients is not only the benefit of symptom relief but also to prevent endovascular treatment or open surgery. Our study was therefore particularly interested in determining whether participation in this long-term WEP at a low-intensity level influenced the need for endovascular or surgical therapies. The results indicated that patients of the usual-care group subsequently underwent significantly more endovascular or surgical procedures than the WEP patients over the years. Although endovascular therapies are recommended for patients with intermittent claudication when they do not respond to initial exercise and medical therapies [16,17] in practice, many patients prefer primarily invasive surgery when given the option. Their heuristics in regard to invasive therapy are based on a low threshold for complications of endovascular revascularization [16]. The immediate benefit of better walking ability after a short invasive procedure rather than elaborate long-term walking exercise training appears to be an attractive alternative to them. The time required to complete a WEP is one of the most common barriers to participation cited by patients who decline [18,19]. Therefore, the most commonly used initial therapy for intermittent claudication is endovascular revascularization [20] . Although they would clearly benefit from it, they do not change physical activity behavior after revascularization [21-23]. Claudicating patients’ risk attitude is correlated to their walking ability and quality of life [24]. Reasons for the association of WEP participation with fewer endovascular or surgical revascularizations could not be discerned from the data presented here.
This study yielded a statistically longer survival time of WEP participants. The walking exercise of the WEP in our study does not meet the recommended intensity of SET but is certainly more effective for the individuals than receiving a simple instruction to walk in their community. Because we did not determine metabolic equivalents of participants during the WEP program, the training intensity delivered in our WEP can only be estimated. With about 60 to 120 min of physiotherapist-delivered exercise training weekly we would assign the training performance of our PAD patients to the low-intensity exercise level. The question arises whether such a low level of exercise can ever lead to positive effects on survival time? Although there is still uncertainty concerning the optimal amount of regular physical activity which reduces an individual's mortality, a dose-response curve of physical activity is scientifically accepted [25]. Garg et al. demonstrated in a long-term study over 57 months that higher levels of physical activity are associated with lower mortality in persons with PAD [26]. Similarly, Gardner et al. described an inverse correlation between the amount of weekly physical activity and mortality rates in claudicating patients [27]. The molecular, cellular, and functional changes that occur during physical activity and lead to a reduction in mortality in the PAD population are still not completely known [28]. Aside from possible improvements in cardiorespiratory fitness [29] however, positive psychological effects [30] and changes of health behavior (e.g., smoking cessation) of WEP participants should not be underestimated. For example, a brief psychological intervention resulted in a sustained change in walking behavior and a reduced rate of revascularizations [31].
The relationship between WEP participation and higher life expectancy seen in our study has an observational character and therefore cannot establish causality. Novel approaches designed to increase community-dwelling physical activity of patients with intermittent claudication should be evaluated. Implementation and evaluation of structured home-based or community exercise programs are promising in respect of their efficacy and costeffectiveness. Currently, for this purpose a prospective randomized study (MOSAIC) has been designed that investigates the concept of a home-delivered physiotherapist-led behavior-change intervention [32,33].
Several limitations exist in this study. First, there is the disadvantage of a single-center study with all its restrictions. A second limitation is that the retrospective study design generates missing values, e.g. as a result of death or departure, which may cause a bias distortion, particularly with respect to confounding atherosclerotic risk factors. Furthermore, data on medical management of study participants were not documented completely, limiting our ability to assess the extent to which optimal medical therapy was used. Finally, because only a minority accepted the recommendation to take part in WEP, participants represent a selected population who conceivably practice a different lifestyle from the non-participants.
Structured long-term WEP, as conducted in our community, is an efficacious therapy in improving and stabilizing the walking ability of PAD patients. Even when patients are trained at a low-intensity exercise level of WEP, their participation is related to fewer endovascular and surgical therapies and higher life expectancy compared to usual care patients. These findings should elicit prospective randomized studies to objectify effectiveness and appropriate dosage of walking exercise which is completed in structured home-based exercise programs on a community level.
We thank the physiotherapists Gudrun Diefenbacher-Ganzhorn and Axel Ganzhorn for gathering and providing the data from the WEP database. Conflicts of interest do not exist.
The authors declare no potential conflicts of interest regarding the publication of this paper.
Citation: Müller-Bühl K, Senft J, Szecsenyi J, Laux G (2020) Long-term Physical Exercise in Patients with Intermittent Claudication. Angiol Open Access. 8:236. doi: 10.35248/2329-9495.20.8.236
Received: 26-Dec-2019 Accepted: 09-Jan-2020 Published: 16-Jan-2020 , DOI: 10.35248/2329-9495.20.8.236
Copyright: © 2020 Müller-Bühl K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.