Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Case Report - (2021)
Toxic Optic Neuropathy (TON) is one of the important causes of bilateral vision loss, decreased color vision and decreased visual acuity. There are several drugs implicated in causing toxic optic neuropathy. We report a case of linezolid induced optic neuropathy in a 45 years old extensively drug resistant pulmonary tuberculosis patient.
Toxic optic neuropathy; Visual acuity; Linezolid; Multidrug Resistant-Tuberculosis (MDR-TB); Extensively Drug Resistant Pulmonary Tuberculosis (XDR-TB)
Linezolid is a member of anti-microbial class known as oxazolidinones. Its spectrum of activity exists against a variety of organisms including the multidrug-resistant microbes like Methicillin Resistant Staphylococcus aureus (MRSA), Vancomycin Resistant Enterococcus spp. (VRE) and drug resistant Mycobacterium tuberculosis [1].Though well tolerated, linezolid is also associated with some serious adverse drug reactions like lactic acidosis, peripheral and optic neuropathy [2]. We report a case of Toxic Optic Neuropathy (TON) due to linezolid occurring in a patient who was on concurrent linezolid and ethambutol therapy for extensively Drug-Resistant Pulmonary Tuberculosis (XDR-TB).
A 45-year-old man presented to the TB and Chest Outpatient Department (OPD) with painless progressive diminution of vision both eyes for the past 20 days. The patient was a known case of XDR-TB who was on treatment with linezolid (600 mg/ day), ethambutol (800 mg/day), pyrazinamide (1250 mg/day), cycloserine (500 mg/day), ethionamide (500 mg/day) and kanamycin (750 mg/day) for the past 20 days. He had no history of smoking, alcoholism and any other substance abuse. On examination, his Best Corrected Visual Acuity (BCVA) was 6/36 in both the eyes, not improving with pin hole. Ishihara color test revealed defective color vision in both eyes. Anterior segment examination was unremarkable and pupils were 2.8 mm, round, regular, and reacting to light in both eyes (Direct and Indirect). Intra-ocular pressure in both the eyes was 18 mm Hg. Fundus examination revealed hyperemic disc with blurred margins in both the eyes. The optic discs showed prominent telangiectatic vessels in both eyes (Figure 1).
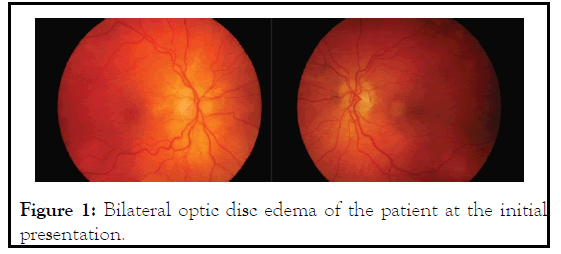
Figure 1: Bilateral optic disc edema of the patient at the initial presentation.
Visual field evaluation by Humphrey field analyzer showed peripheral constriction in the right eye and low reliable fields in the left eye. Contrast vision was reduced in both the eyes. Optical Coherence Tomography (OCT) revealed increased Retinal Nerve Fiber Layer (RNFL) thickness in both eyes. Originally, ethambutol-induced optic neuropathy was initially thought and which was discontinued after discussion with the treating physician. Even after 15 days of discontinuing ethambutol, the patient's visual acuity progressively dropped to 6/60 in both eyes, and the fundus remained unchanged. Hence, TON due to linezolid was considered. The causality assessment of the adverse drug reaction came out to be possible for this case using the Naranjo Causality Assessment Scale [3]. The case was reported to the ADR monitoring center under the Pharmacovigilance program of India (PvPI). Rapid improvement in visual acuity was seen 15 days post discontinuation of linezolid. Color vision was completely restored to normal and patient's vision was restored at 6/6 after 45 days of discontinuation of the linezolid therapy. Fundus examination revealed resolved optic disc edema with imminent temporal pallor in both eyes. Follow-up of visual field showed partial improvement and the OCT demonstrated normalization of RNFL. Tablet ethambutol was restarted at the insistence of the physician. The patient is under regular follow-up and no toxic effects have been noted at three months of follow-up.
Toxic optic neuropathies are characterized by gradual, progressive, painless, bilaterally symmetric visual loss affecting central vision, associated with causing central or centrocecal scotomas. TON may result from exposure to a neuro-toxic substance, elevated serum drug levels leading to impairment in the tissue’s vascular supply. Many vitamins including thiamine (B1), riboflavin (B2), niacin (B3), pyridoxine (B6), cobalamin (B12), folic acid and proteins are also be implicated in the causality of TON. Among the multifactorial etiology of TON the common causes include the ingestion of tobacco, methanol, disulfiram, halogenated hydro-quinolones, and antibiotics such as ethambutol, isoniazid, chloramphenicol, sulphonamides and linezolid beside others [4].
The cornerstone in the management of TON remains the discontinuation of the offending medication and conducting repeated follow-up of the patient to observe the progression of the disorder. Patients with TON should be assessed every 4-6 weeks for pupils, optic nerves, color vision visual acuity and visual fields at each follow-up visit. Vision gradually recovers to normal over a period of several weeks, although it may take few months for complete recovery. Usually, visual acuity has been seen to recover before an improvement in the color vision is appreciated [4].
Extensively Drug-Resistant TB (XDR-TB) is defined as a type of Multidrug-Resistant Tuberculosis (MDR-TB) that is resistant to isoniazid and rifampin, plus any fluoroquinolone and at least one of three injectable second-line drugs (i.e. amikacin, kanamycin, or capreomycin) [5].
Linezolid inhibits protein synthesis by preventing formation of the ribosome complex which initiates protein formation. Its unique binding site located on 23S ribosomal RNA of the 50S subunit results in no cross resistance with other drug classes. Linezolid has been used in the treatment of infections caused by multidrug-resistant Gram-positive bacteria [2]. Though well tolerated linezolid is usually well tolerated, some serious adverse drug reactions have also been reported with its use including myelosuppression including anemia, thrombocytopenia, and leukopenia, and lactic acidosis, peripheral and optic neuropathy [6]. There are very few case reports of linezolid-induced optic or peripheral neuropathy in patients following short term linezolid treatment of less than 28 days [7]. The most common indication for long-term linezolid therapy in these patients has been infection with methicillin-resistant Staphylococcus aureus vancomycin-resistant Enterococcus faecium infections [8]. In this case, neuropathy occurred after linezolid had been used 20 days at a dose of 600 mg per day for infection with Mycobacterium tuberculosis. Toxic optic neuropathy was attributed to linezolid in the patient as even after the discontinuation of drug there was a progressive deterioration of vision, which improved only after withdrawal of linezolid. Linezolid is used to treat drugresistant tuberculosis as a part of combination therapy [9]. Linezolid may improve the chance of bacteriological cure only in the most complicated XDR-TB cases [10]. XDR-TB, where there is a scarcity of treatment options, the treating physicians are compelled to resort to alternate drug therapies [11].
With our country having very high number of new and resistant tuberculosis patients, for which drugs from various classes are used in the treatment. Hence, it is important for the treating physician to keep in mind the possible long-term effects of different drugs and enquire about them from the patient at regular follow-ups for early recognition of possible toxicity and discontinuation of drug.
Citation: Khanna S, Pant S, Khanna H (2021) Linezolid Induced Toxic Optic Neuropathy in Patient of Extensively Drug Resistant Pulmonary Tuberculosis. J Clin Exp Ophthalmol.12:900.
Received: 01-Dec-2021 Accepted: 15-Dec-2021 Published: 22-Dec-2021
Copyright: © 2021 Khanna S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.