Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2014) Volume 5, Issue 2
The mosquito Anopheles not only cause nuisance by their bites but also transmit deadly diseases like malaria in Sub-Saharan Africa, where most of the malaria deaths occur in the world. In Ethiopia, despite the use of native plants in traditional combat against mosquitoes, use in a modern way has remained scanty. The present study, being based on an initial ethno botanical survey, carried out screening experiments on five indigenous ethno botanical species (Aloe pirottae Berger, Aloaceae; Acokanthera schimperi (A.DC) Schweinf, Appocynaceae; Brassica nigra L. Koch, Brassicaceae; Oreosyce africana Hook.f., Cucurbitaceae and Piper capense L.f., Piperaceae). The larvicidal activity of 80% methanol extracts of the first two plant species against the fourth instars of Anopheles arabiensis Patton, and adulticidal activity with the same solvent extracts of the latter four species against Anopheles arabiensis adults, gave positive results upon evaluation under laboratory condition. The 80% methanol extract of the gel of A. pirottae had more activity within 24 hours on the larvae than the leaf extract of Acokanthera schimperi. The highest (100%) mortality in the fourth instars occurred on treatment with 160 ppm extract of Aloe pirottae and 480 ppm extract of Acokanthera schimperi. The maximum adult mortality was detected in the leaf extract of Oreosyce africana (LC50 18.74 and LC90 39.66 ppm) followed by fruit extract of Piper capense (LC50 24.30 and LC90 46.32 ppm), while no mortality was noticed in the control groups. Phytochemical screening of the methanol extracts of the leaves of Oreosyce africana and the fruits of Piper capense had key secondary metabolites (alkaloids, saponins, flavonoids, cardiac glycosides), further corroborating their adulticidal properties. These findings announce the first evidence that Aloe pirottae is a promising mosquito larvicide while Oreosyce africana and Piper capense carry huge potentials as mosquito adulticides contributing to integrated malaria control through proper mosquito management.
<Keywords: Indigenous plant extracts; Anopheles arabiensis; Larvicide; Adulticide
Vector borne diseases are among the major causes of illness and death in many developing countries. Mosquitoes (Diptera: Culicidae) are responsible for transmitting the most important vector borne diseases including malaria, lymphatic filariasis, Japanese encephalitis, and dengue as well as yellow fever and other forms of encephalitis [1]. Malaria is now responsible for illness in more than an estimated 300 million people, resulting in one million deaths per year [2]. Malaria is transmitted through bites of parasite-infected Anopheles mosquitoes. The abbreviation for Anopheles arabiensis is An. arabiensis [3], which is the most important vector associated with the transmission of malaria disease. Anopheles arabiensis is the major malaria vector in Ethiopia [4-6]. The approach to combat this disease largely relied on interruption of the disease transmission cycle by either targeting the mosquito larvae through spraying of stagnant waters that serve as breeding sites or by killing the adult mosquitoes using insecticides. The malaria control strategies in Ethiopia include the combination of insecticide treated nets and indoor residual spraying that was found to be effective as a control measure [7].
Mosquito resistance to the currently-used insecticides and the emergence of multi drug-resistant strains of parasites has escalated the malaria problem in the affected countries. According to the studies conducted in Ethiopia [6,8-10], An. arabiensis was resistant to an array of insecticides, including DDT, permethrin, deltamethrin and malathion. Earlier report [11] has documented mosquito resistance to the four classes of insecticides. Thus, synthetic insecticides have created several problems including the development of resistant insect strains, ecological imbalance and harm to mammals. These drawbacks of the hitherto developed insecticides, researchers in the area are working hard to find environmentally safe alternatives. Botanical insecticides may serve as suitable alternatives to synthetics in future, as they are relatively safe, degradable and readily available in many parts of the world [12]. For this, they resorted to plant extracts as potent sources of natural biocides [13].
Although insecticides of plant origin have been extensively used on agricultural pests, a very limited extent has been used against insect vectors of public health importance [14]. Because of these many of the reported tropical plants came under scrutiny, leading to extraction and characterization of their active constituents, which accounted for various uses by people. Among the most important constituents are alkaloids, terpenoids, steroids, phenols, saponins and tannins [15]. Mosquito controls strategies; especially those that are effective, cheap and environmentally non-hazardous are needed. Hence, crude plant extracts have played an important role in this aspect. One of the most studied botanical species with good pesticides attributes is the neem tree (Azadirachta indica A. Juss.), whose extracts have shown considerable activity and multiple modes of action against agricultural pests, forestry insects, and insects of public health [16].
Many herbal products have been used as natural insecticides before the discovery of synthetic organic insecticides [17]. And a wide range of plants with larvicidal and adulticidal properties have been used against insects [18]. In Ethiopia, about 1000 higher plants are employed in the traditional healthcare and that 60% of them are said to be indigenous and the same proportion is believed to have healing potentials [19]. Prevention of malaria and other diseases is commonly practiced by indigenous people using traditional insecticides and insect repellent plants. For example, a study conducted by Berhanu et al. [20] showed that Allium sativum Linn is the most widely used mosquito repellent followed by Lepidium sativum L. and Capparis tomentossa Lam. In addition to being readily available in different parts of the country, locally growing plants are economically more viable and the methods employed are usually simpler. Though several compounds of plant origin have been reported as insecticides and larvicides, there is still a wide scope for the discovery of more effective plant products [21] particularly in the indigenous flora of lesser studied countries like Ethiopia.
In the present investigation, extracts from indigenous Ethiopian plants have been tested to assess their larvicidal and adulticidal properties when applied to the larvae and adults of An. arabiensis, an important malaria vector in Ethiopia. The gel extract of Aloe pirottae (A. pirottae) and the leaf extract of Acokanthera schimperi (A. schimperi) were checked for efficacy as a larvicidal agent. Likewise, four plant species (viz., the leaf extracts of Oreosyce africana (O. africana) and gel extracts of A. pirottae, seed extracts of Brassica nigra (B. nigra) and fruit extracts of Piper capense (P. capense) were checked for its efficacy as an adulticide against the same vector.
Selection and collection of plant material
For the present study, five different plant species were selected from different regions of Ethiopia. Plant selection in the study was based on interviewing traditional herbalists and members of the community to specify the indigenous plant species known for their use in the regular control of mosquitoes and other insects in their localities, and through analysis of the relevant literature. To select the most relevant and promising species for purpose of chemical screening, all the candidate plants were ranked following the application of weighted criteria as described by Martin [22]. By combining the ethno botanical leads and chemotaxonomic evidence (popularity of plants already used as insecticides by local people and documented evidence of insecticidal constituents in the family to which the candidate species belongs) highly promising plant species are selected for testing. Following such procedure provided useful information essential for further evaluation of larvicidal and adulticidal activities of the few priority plants tested against An. arabiensis. Once the priority plants are identified, the next step involves isolating and identifying the new bioactive chemical compounds present in the indigenous plant species that might possess superior larvicidal/adulticidal properties.
Plant material collection
Plant materials of the five indigenous plant species listed in Table 1 were collected from different localities in Oromia Region, Ethiopia, and kept separately in plastic bags and brought to the laboratory for extraction. The collections were made in September and November 2011 and were authenticated by Dr. Zemede Asfaw, Prof. Ensermu Kelbessa, and Mr. Melaku Wondafrash, Department of Plant Biology and Biodiversity Management, Addis Ababa University, Ethiopia. For future reference, the voucher specimens have been deposited at the National Herbarium (ETH), Addis Ababa University.
| Voucher No | Scientific name | Vernacular name (Afan Oromo) | Family name | Plant habit | Locality/ | Part used | Altitude (m) | Long (N) | Lat (E) |
|---|---|---|---|---|---|---|---|---|---|
| District | |||||||||
| DB38 | Acokanthera schimperi (A.DC.) Schweinf. | Qararuu | Appocynaceae | Tree | Tapisa Medale/ Gindeberet | Leaf | 1955 | 09045.458' | 037045.687' |
| DB37 | Aloe pirottae Berger | Hargissa | Aloaceae | Herb | Iftu/ Babile | Leaf/Gel | 1313 | 09012.494' | 0420 15.228' |
| DB27 | Brassica nigra L. Koch | Shinfii | Brassicaceae | Herb | Yerer lencho/ Akaki | Seed | 1930 | 08047.144' | 038053.712' |
| DB18 | Oreosyce africana Hook.f. | Manabasi | Cucurbitaceae | Herb | Yerer lencho/ | Leaf | 1936 | 080 50.682' | 0380 56.630' |
| Akaki | |||||||||
| DB22 | Piper capense L. f. | Tunjo | Piperaceae | Herb | Bada Buna/ | Fruit | 1779 | 07039.730' | 036053.213' |
| Asendabo |
Table 1: Plant materials collected from different localities of Ethiopia in September and November 2011.
Preparation of extract
The leaves of A. schimperi and O. africana, seeds of B. nigra and fruits of P. capense were all shade dried, ground and sieved to get fine powder from which the extracts were prepared. The gel of A. pirottae was collected by cutting the fresh leaves using a razor blade and gathered into glass vials. All these plants were extracted in a separate container in 80% methanol (SCP, England), using Erlenmeyer flasks and placed on orbital shaker (VWR, U.S.A.) by maceration or cold extraction method. After leaving the methanolic solution to rest for an overnight, it was filtered through Whatman no. 40 filter paper (Whatman International Ltd., Maidstone, England) and the filtrate was collected. This procedure was repeated three times with fresh volume of methanol. The filtrates were pooled and the pooled extracts were concentrated using a vacuum evaporator below 45°C under low pressure and over water bath until the solvent completely evaporated. The extract of each plant thus obtained was lyophilized and then placed in the freezer at -20°C until testing for mosquito larvicidal and adulticidal activity. Standard stock solutions were prepared at 1% and from the stock solution various concentrations were prepared.
Rearing of Anopheles arabiensis
Anopheles arabiensis, a potent malaria vector of Ethiopia, was selected for laboratory evaluation. Eggs were obtained from a mosquito colony maintained at the insectarium of the Ethiopian Health and NutritionResearch Institute, which had been reared according to the standards of the World Health Organization [23]. The colonies were established and maintained at the insectarium of College of Natural Sciences or the Department of Zoological Sciences, Addis Ababa University at optimum conditions (25-27°C temperature and 70-80% relative humidity). For oviposition, glass petri dishes with wet filter paper were placed inside the cage, which were then transferred to tray with distilled water for egg hatching. Larvae were reared in tray and fed on ground Tetramin® fish food pellets (Tetra holding Inc., Blacksburg, VA, U.S.A.). Water of the larval culture was changed every third day to avoid decay. Pupae were transferred to cup by disposable pipettes and put in screened cages for adult emergence. The adults were fed on 10% glucose solution soaked in cotton pads. Rabbit with shaved back and belly was provided for blood meal for the female mosquitoes. By doing so, 4th instar larvae and adults were continuously available for the different bioassay tests.
Larvicidal bioassay
The larval bioassay tests for the larvicidal effect of 80% methanolic extract derived from A. pirottae gel and A. schimperi leaf against An. arabiensis were carried out in accordance with the WHO standard method [24-25] with little modifications. Each of these desired concentrations added to 149 ml deionized water in 200 ml capacity plastic cups. The test concentrations of 40, 80, 120, 160, 240, 360, and 480 ppm of the plant serial dilution prepared extract in deionized water. Tween-80 (0.02%) as emulsifier (Tween-80, Sigma, USA) was added with the extracts of A. pirottae and A. schimperi to ensure complete solubility of the material in water. Fourth instar larvae of An. arabiensis were placed in each test solution as in the above mentioned concentration. Tests were carried out, in three replications, simultaneously using four batches of 20 larvae (a total of 80 larvae) and one batch as untreated control group for each concentration and the setup of the test was assigned in a completely randomized design [26]. For each test three cups containing equal amount of deionized water and Tween-80 and test larvae but without sample were used as controls. Observation on mortality of the larvae was recorded after 24 hours of continuous exposure and this was expressed as percent mortality. Dead larvae were identified when they failed to move after probing with a needle in the siphon or cervical region. Dead larvae were removed as soon as they were discovered to prevent decomposition, which may cause rapid death of the remaining larvae.
Adulticidal test
Laboratory studies have been carried out to determine the adulticidal properties of the candidate plant species against An. arabiensis. The required concentrations of different plant extracts (concentrations of 6.25, 12.5, 25, 50, 100, and 200 ppm) were prepared through the mixing up of stock extract with variable amounts of sterilized distilled water and dimethyl sulphoxide (DMSO) was used as a wetting agent at a 0.05% concentration in the final test solution of the extract. The solution was impregnated on filter papers (diameter 6.5 cm) with the help of the pipette in plastic container and untreated net covering the beaker was also impregnated. Equal amount of solvent (sterilized distilled water) and DMSO was added on the filter paper without the extract and kept as control. The papers were allowed to dry overnight at room temperature under shade before testing.
Then the dried filter papers were placed in 250 ml glass beakers (Solidex, France) similar to the Centers for Disease Control and Prevention (CDC) glass bottle bioassay of insecticides [27] but with slight modifications. A total of 20 laboratory reared adult female An. arabiensis were gently aspirated into each beaker using a mouth aspirator. The treatment on each concentration was replicated three times in a completely randomized design [26]. The triplicate series contained 20 females in each beaker. Therefore, a total of 80 mosquitoes were assayed for each plant extract and one batch as negative control group for each concentration. The same protocol was applied to negative control experiments in which mosquitoes were aspirated into beakers containing filter papers soaked in sterilized distilled water using 0.05% DMSO and dried in the same condition as for the extracts. Adult food, 10% sugar solution was provided to both the experimental and the control adults. Mortality was recorded at 24 hour intervals. Mosquito mortality was recorded as dead if it was lying on its back or side and was unable to maintain flight after a gentle tap on the bottle. For 55 ppm concentration of O. africana and P. capense, mortality counts were also undertaken after 2, 4, 8, 12, and 24 hours and expressed as percentage mortality.
Percentage mosquito mortality was calculated by using the formula:
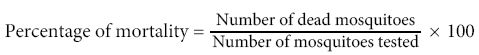
Methods of Preliminary Phytochemical screening of Oreosyce africana and Piper capense
Preliminary qualitative screening of major secondary metabolites of the extract of O. africana and P. capense were conducted. The methods employed, include: Dragendroff's/ Mayer's for alkaloid, Modified Borntrager's for glycosides, froth and foam for saponins, ferric chloride (FeCl3) for phenols, Gelatin for tannins, Xanthoproteic and Ninhydrin for protein and amino acid, Salkowski's and Liebemann-Burchard's for phytosterols were used to determine the presence of specific phytochemical groups using the methods described in the relevant literature [28-30]. The reagents used for screening were Hydrochloric acid (Riedel-de Haen, Hannover, Germany), Potassium iodide (Evans Medical Limited, Liverpool, UK), Sulfuric Acid (Merck, Germany), Ammonia solution (Park Scientific, Northampton, UK), Nitric Acid (Aldrich chemical Co. Ltd, England), Acetic anhydride (Riedel-de Haen, Hannover, Germany), Sodium hydroxide pellets (Park scientific, Northampton, UK), and Sodium Chloride (Parker, England). All the reagents were analytical grade.
Data analysis
All experiments were done in triplicate, larvae and adults mortality counts were made after 24 hour exposure, whereby mortality between 10 and 100% was considered. Bioassay tests showing more than 20% control mortality were discarded and repeated. However, when control mortality ranged from 5% to 20%, the corrected mortality was calculated using Abbott's formula (Abbott, 1925) as shown below. Mean percent mortalities of the adult An. arabiensis that were treated with crude methanol leaf extracts were determined by one-way analysis of variance (ANOVA) using the SPSS for windows version 16.0, SPSS software 2007 [32]. The concentration in ppm lethal to 50% (LC50) and 90% (LC90) for larvicidal and adulticidal with its 95% confidence intervals of upper confidence limit (UPL) and lower confidence limit (LCL), chi-square values, the slope, and the degrees of freedom were calculated using the POLO PLUS version 2.0, LeOra Software 2007 [33]. Results with P<0.05 were considered to be statistically significant.
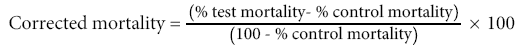
The description of the five plant species studied and the percent yields of 80% methanol extract are as given in Table 2.
| S. No | Plant species and families | Net weight (g) | Yield of extract (%) |
|---|---|---|---|
| 1 | Acokanthera schimperi (Appocynaceae) | 596 | 15.1 |
| 2 | Aloe pirottae (Aloaceae) | 805 | 11.4 |
| 3 | Brassica nigra (Brassicaceae) | 557 | 12.8 |
| 4 | Oreosyce africana (Cucurbitaceae) | 1700 | 39.0 |
| 5 | Piper capense (Piperaceae) | 1800 | 37.2 |
Table 2: Plant materials investigated for larvicide and adulticide against Anopheles arabiensis with their net weight and yield of the extract.
Larvicidal activity of the plant extracts against fourth instars larvae of An. arabiensis
Extracts of A. pirottae gel and the leaves of A. schimperi gave 100% mortality at 160 and 480 ppm, respectively against An. arabiensis larvae as shown in Figure 1. There was no mortality observed in the control group.
- Concentration (ppm, part per million)
As shown in Table 3, the extracts of A. pirottae and A. schimperi tested on An. arabiensis larvae within 24 hours gave the median lethal concentration (LC50) values of 76.34 and 282.76 ppm, respectively.
| Plant extracts | 95% confidence limit | LC90 (ppm) | 95% confidence limit | χ2 (df =4) | Slope + SE | |||
|---|---|---|---|---|---|---|---|---|
| LCL | UCL | LCL | UCL | |||||
| Aloe pirottae | 76.344 | 31.47 | 105.42 | 133.39 | 104.59 | 249.5 | 3.7109* | 0.022 ± 0.003 |
| Acokanthera schimperi | 282.76 | 135.1 | 338.93 | 407.93 | 399.19 | 610.02 | 6.6342* | 0.01 ± 0.001 |
Table 3: Larvicidal activity of methanolic extract of the two local plants, Aloe pirottae and Acokanthera schimperi against fourth instar larvae of Anopheles arabiensis with its respective lethal concentrations, confidence intervals (95%), chi-square, slope and standard error after 24 hour of exposure
Effect of plant extracts on Anopheles arabiensis adults
The results of the mean mortality in adult An. arabiensis samples upon application of 80% methanol extracts of A. pirottae, B. nigra, O. africana and P. capense are presented in Table 4. Among the four plants tested, the highest adulticidal activities were observed in the extracts of O. africana and P. capense. The LC50 and LC90 values were 18.74, 39.66 ppm and 24.30, 46.32 ppm, respectively (Table 5). However, in the extract of A. pirottae the LC50 and LC90 values were 176.81 and 319.80 ppm, respectively which shows that the highest value of lethal concentration is needed among the four plant extracts. The 95% confidence limits [LC50 (LCL-UCL)] and [LC90 (LCL-UCL)] were also calculated (Table 5). No mortality was recorded in the control. The results clearly show that the percentage of mortality is directly proportional to the concentration of the extract. The chi-square values are significant at P<0.05 level.
| Plant extracts | % mortality (M+SD) | Order of relative efficacy | |||||
|---|---|---|---|---|---|---|---|
| 6.25 ppm | 12.5 ppm | 25 ppm | 50 ppm | 100 ppm | 200 ppm | ||
| Aloe pirottae | 0.00 ± 0.00 | 3.33 ± 2.88 | 11.67 ± 2.88 | 20.00 ± 5.00 | 30.00 ± 5.00 | 53.33 ± 5.77 | 4 |
| Brassica nigra | 8.33 ± 2.88 | 8.33 ± 2.88 | 25.00 ± 0.00 | 28.33 ± 2.88 | 35.00 ± 5.00 | 75.00 ± 5.00 | 3 |
| Oreosyce africana | 20.00 ± 5.00 | 46.67 ± 2.88 | 51.67 ± 2.88 | 100.00 ± 00 | 100.00 ± 00 | 100.00 ± 00 | 1 |
| Piper capense | 15.00 ± 5.00 | 28.33 ± 2.88 | 45.00 ± 5.00 | 95.00 ± 00 | 100.00 ± 00 | 100.00 ± 00 | 2 |
M = means of three replicates, SD = standard deviation, *no mortality was observed in controls
Table 4: Adulticidal activity of various plant extracts applied for 24 hours on female Anopheles arabiensis.
| Plant extracts | LC50 (ppm; LCL-UCL) | LC90 (ppm; LCL-UCL) | χ2 (df=4) | Slope ± SE |
|---|---|---|---|---|
| Aloe pirottae | 176.817 (129.560 - 307.093) | 319.802 (229.932 - 628.162) | 1.787* | 1.564 ± 0.206 |
| Brassica nigra | 128.654 (97.353 - 185.462) | 264.787 (201.813 - 416.425) | 10.517* | 1.298 ± 0.160 |
| Oreosyce africana | 18.743 (10.974 -27.735) | 39.663 (29.823 - 72.346) | 20.935* | 2.691 ± 0.270 |
| Piper capense | 24.305 (21.120 - 27.903) | 46.327 (40.722 - 54.782) | 15.920* | 2.854 ± 0.264 |
Table 5: Adulticidal effect of methanolic extract of the four local plants against Anopheles arabiensis with its respective lethal concentrations, confidence intervals (95%), chi-square, slope and standard error after an exposure of 24 hours. LC50: lethal concentration that kills 50% of the exposed adults; LC90: lethal concentration that kills 90% of the exposed adults; LCL: Lower Confidence Limit; UCL: Upper Confidence Limit; χ2: Chi-square; df: degree of freedom; ±SE: Standard Error. *Significant at P <0.05 level
Among the extracts, O. africana and P. capense showed more remarkable adulticidal potential than A. pirottae and B. nigra, presenting LC50 and LC90 values of 18.74 and 39.66, and 24.30 and 46.32 ppm, respectively (Figure 2). No mortality was observed in control.
Phytochemical screening of Oreosyce africana and Piper capense
The results of preliminary qualitative phytochemical analysis of tested plant crude extract revealed the presence of some secondary metabolites which may be active ingredients for adulticidal activity (Table 6).
| Plant constituents | Plant extract | |
|---|---|---|
| Oreosyce africana (leaves) | Piper capense (fruits) | |
| Alkaloids | + | + |
| Phenols | + | + |
| Tannins | - | - |
| Phytosterols | + | - |
| Triterpenes | + | - |
| Flavonoids | + | + |
| Saponins | + | + |
| Glycosides | + | + |
| Phenolic glycoside | + | - |
| Cardiac glycosides | + | + |
| Free anthraquinone | - | + |
| Anthranoids | - | - |
| Chromophers | + | + |
| Protein and amino acid | - | - |
Key: + = present; - = absent
Table 6: Preliminary phytochemical screening results for Oreosyce africana and Piper capense crude extracts.
In both species, most constituents were found to be alkaloids, phenols flavonoids, saponins and chromophers while tannins, anthranoids, protein and amino acids were not detected. Phytosterols, triterpenes, and phenolic glycoside were detected in the extract of O. africana but not found in P. capense. Free anthraquinone was found in P. capense. Such preliminary phytochemical screening gives a brief idea about the qualitative nature of phytoconstituents present in the leaf and fruit extracts of O. africana and P. capense, respectively.
The present study, which started with an ethnobotanical survey, was follow-up by laboratory investigation on plants that turned out strong leads to protection against insects. Thus, the methanol extract of A. pirottae gel (LC50 = 76.34) showed biological activity against the fourth instar larvae of An. arabiensis. This is comparable to the methanol leaf extract of Ocimum canum Sims (LC50=72.40 ppm) as reported by Bagavan et al. [34]. The methanol leaf extract of A. schimperi (LC50 = 282.76 ppm) is potent against the larvae of An. arabiensis. This finding agrees with that of Patil et al. [35] who have tested the methanol root extract of Balanites aegyptica L. (Del.) against Ae. aegypti Linn larvae and was found to be the median lethal concentration (LC50 = 289.59 ppm). Clearly, the extracts of both the plants are highly effective in killing the mosquito larvae, thereby showing its toxic property. The results of larvicidal activity showed that the percentage of mortality in larvae increases with the increase in the concentration of the extract and is concentration dependent.
The activity of crude plant extract is often attributed to the complex mixture of active compounds. Therefore, the preliminary screening is a good means of evaluating the potential larvicidal and adulticidal activity of plants popularly used for this purpose in the traditional medical systems of local communities. Larvicidal activity of crude solvent extracts of two plants and adulticidal activity of four plants are noted and presented in Tables 3 and 5. Only those plants that showed a LC50 value of less than 100 ppm for both larvae and adult mosquitoes were considered as efficient mosquitocidal plants. Based on observations made in the 24 hour bioassay studies, A. pirottae against the Anopheles larvae and O. africana and P. capense against adult Anopheles, showed a LC50 value of less than 100 ppm and were identified as efficacious plants against them.
Aloe pirottae is a species endemic to Ethiopia [36]. The extract from A. pirottae is reported for its current use to treat tropical ulcer, eye diseases, malaria, snake bite, gastro-intestinal parasites and gallstone and for cleaning the human colon in eastern Ethiopia [37]. Another finding reported by Matasyoh et al. [38] showed that the extract of Aloe turkanensis Christian, at a concentration of 0.2 mg/ml, resulted in 100% larval mortality of An. gambiae Giles and had a LC50 of 0.11mg/ml. According to the report [39], the LC50 and LC90 values of methanol leaf extracts of Croton macrostachyus Del were 89.25 and 224.98 ppm, respectively against late third instar larvae of malaria vector, An. arabiensis. In another study, the leaf methanol extract of Cassia fistula L. was tested for larvicidal activity against Cx. quinquefasciatus Say and An. stephensi Liston, which exhibited the LC50 values of 17.97 and 20.57 mg/l, respectively [40].
According to a study conducted by Kumar and Maneemegalai [41], the methanol extract of leaves of Lantana camara Linn. (Family: Verbenaceae) showed 100% in 4th instar larvae of Cx. quinquefasciatus. Evidently the extracts of A. pirottae and A. schimperi are highly effective in killing the mosquito larvae, thereby showing the toxic properties of these species. Acokanthera schimperi is also mentioned for use against mosquito and malaria in eastern Ethiopia [37]. In the bioassay larval test with the two plant species on An. arabiensis, mortality in larvae increased with the increase in the level of concentration, similar to an earlier report [42]. Methanolic extract of the leaves of Atlantia monophylla Corr. (Rutaceae) were evaluated for mosquitocidal activity against the immature stages of mosquitoes, Cx. quinquefasciatus, An. stephensis and Ae. aegypti in the laboratory [13]. On the water treated with Citrullus colocynthis (L.) Schrad (Family: Cucurbitaceae) extract at 100 ppm concentration some dead adult female mosquitoes were observed [43].
The application of botanicals for the control of mosquitoes is recommended [44] because they minimize the accumulation of harmful residues in the environment. The crude extracts may be more effective compared to the individual active compounds, due to natural synergism that discourages the development of resistance in the vectors [45]. A study conducted in the Awash river valley of Ethiopia has showed that the female An. arabiensis tend to avoid dichloro-diphenyl-trichloroethane sprayed surfaces and thus enables its population at a sufficient level to sustain transmission [4]. The present results have showed that crude methanol extract of the gel of A. pirottae have significant larvicidal activity compared to its adulticidal activity. Plant products can be used, either as insecticides for killing larvae or adult mosquitoes or as repellents for protection against mosquito bites, depending on the type of activity they possess [17]. Since most breeding sites of mosquitoes can easily be detected for spraying, the use of such inexpensive botanical extracts for larval control that will end their life cycle and can safely minimize the multiplication of the mosquito population and its consequent transmission of the malaria disease.
Today, the environmental safety of an insecticide is considered to be of paramount importance. Phytochemicals may serve as suitable alternatives to synthetic insecticides in future as they are relatively safe, inexpensive, and are readily available throughout the world. According to Bowers et al. [46], the screening of locally available medicinal plants for mosquito control would generate local employment, reduce dependence on expensive imported products and stimulate local efforts to enhance public health. The filtrate obtained from O. africana was reported as being applied through hypodermal injection using a syringe to treat gonorrhea [47]. This species is also traditionally used for insecticides [48]. In another study, O. africana was used for anti-malarial treatment [49]. The biological activity of O. africana and P. capense extracts might be due to the various compounds, including alkaloids, phenols, saponins, and cardiac glycosides existing in plants as shown in the present investigation (Table 6). These compounds may jointly or independently contribute to produce adulticidal activity against An. arabiensis. In line with the present investigation, the phytochemicals in methanol extract of Lantana camara var. alba showed the presence of cardiac glycosides, flavonoids, and saponin [41].
Basing on the preliminary screening results, four crude solvent extracts were subjected to bioassay. Out of these, methanol extract of O. africana leaves was found to possess the most effective adulticidal activity (LC50 18.74 ppm) against An. arabiensis followed by methanol extract of P. capense (LC50 24.30 ppm) fruits, methanol extract of B. nigra seeds (LC50 128.65 ppm) and A. pirottae gel (LC50 176.81ppm). A gradient that increases in mortality with increase in concentration was observed in all the treatments. Our results showed that both methanol extract of the O. africana and P. capense have significant adulticidal activity (LC50=18.74 and 24.30 ppm), respectively against An. arabiensis. These results were about 2 and 3-fold more active than the earlier reports of Kamaraj et al. [50] who observed the adulticidal activity of the methanol extract of Azadirachta indica and Justicia procumbens Linn., have adulticidal activity (LC50=37.75 and 85.66 ppm), respectively against Cx. gelidus Theobald.
In our findings, the residual concentration effects of O. africana and P. capense were 95 ppm and 100 ppm, respectively and gave 100% mortality of adult An. arabiensis in the first six hours. However, for 24 hours time spent O. africana (50 ppm) and P. capense (55 ppm) had the residual concentration effect for the 100% mortality as shown in Figure 3. The symptoms observed in adult mosquitoes were similar to those caused by nerve poisons i.e., excitation, convulsion, paralysis and death [51].
Due to the toxic action of O. africana and P. capense against the adults, these extracts show potential to suppress the population of An. arabiensis which constitute a major link between humans and the Plasmodium that causes malaria. Therefore, controlling the adult female Anopheles by using plant products helps for hindering from egg depositing and blocking the malaria transmission. Both O. africana and P. capense grow wild in uncultivated areas and the plant materials could be easily collected without any additional cost, hence these two plants extracts warrant for use to control the population of mosquitoes as an adulticidal agent in an integrated vector control program. However, large scale harvesting from nature will not be sustainable in the long run and hence cultivation in marginal areas could be considered as an option. Further investigations on identification of active ingredient(s) of the extract responsible for adulticidal activity in An. arabiensis are needed for development of eco-friendly chemicals for control of mosquito vectors.
Due to its toxic action against the larvae of An. arabiensis, extracts of A. pirottae show potentiality to suppress the population of An. arabiensis. Both O. africana and P. capense extracts are promising for use in the control of malaria vector, An. arabiensis because of the adulticidal property of the extracts. The present findings have important implications in the practical control of mosquito larvae and adults by using botanical extracts. These plant extracts are easy to prepare, inexpensive, and safe for mosquito control which possess enough potential in larvicide and adulticide and can be used directly as larvicidal and adulticidal agents in small volume aquatic habitats or in/around human dwellings. The results suggest for a possible utilization of the cheap and readily available botanicals for possible control of mosquitoes as part of an integrated vector management program. Results from this study have initiated further investigations into the bioassay-guided fractionation, isolation and purification of compounds from the crude methanol extract of leaves of O. africana and the fruits of P. capense.
The authors wish to acknowledge the Department of Microbial, Cellular and Molecular Biology and Thematic Research of Malaria and other Parasitic Diseases for their financial support. We are grateful to Mr. Yehualashet Belete, the Centre for Traditional Medicine and Drug Research, and Mr. Fitsum Tesfaye, the Centre for Mosquito rearing and maintaining, the staff of Ethiopian Health and Nutrition Research Institute for their technical assistance and for providing necessary research facilities for the present investigations.