
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Review Article - (2020)Volume 10, Issue 3
Diabetes is known to increase the risk of myocardial and cerebral infarction, as well as complications such as retinopathy, nephropathy and neuropathy. It is important for diabetics to improve their eating habits, for example, by eating slowly, avoiding overeating, and increasing their intake of vegetables, in order to suppress sudden rises in blood glucose (Glu) levels. On the other hand, alginic acid (Alg), which is a polysaccharide derived from brown algae, is used as a food and medicinal additive, a health food and a pharmaceutical, based on its blood cholesterol-lowering effect and protective effect on the gastric mucosa. It was recently reported that calcium alginate (Ca-Alg) has the pharmacological effect of suppressing blood Glu elevation. We investigated the mechanism of this effect and found that Ca-Alg inhibits the metabolism of starch in the gastrointestinal tract by inhibiting the activity of α-glucosidase, which degrades maltose into Glu.We also examined the influence of the amount of Ca-Alg added to the diet and the particle size of the Ca-Alg on postprandial blood Glu elevation in rats. Subsequently, we conducted several clinical trials. We asked healthy adults to eat udon noodles, soba (buckwheat noodles), and Chinese noodles containing CaAlg, and measured the blood Glu levels after consumption. In each case, Ca-Alg decreased blood Glu levels and suppressed total absorption of Glu. This review summarizes the results of our basic and clinical studies on the effects of Ca-Alg on postprandial blood Glu elevation.
Calcium alginate (Ca-Alg); Blood glucose level; α-Glucosidase; Noodles; Diabetes; Clinical trial
In recent years, the number of people with lifestyle-related diseases has increased dramatically [1,2]. In particular, the incidences of diabetes, hypertension, and dyslipidemia are increasing, and these diseases increase the risks of cancer, heart disease, and cerebrovascular disease, which are leading causes of death in developed countries [3-10]. For example, diabetes increases the risks of myocardial and cerebral infarction, as well as complications such as retinopathy, nephropathy and neuropathy [11,12]. Unfortunately, diabetes is often diagnosed late, because its initial symptoms are subjective, so prevention is very important.
Glucose (Glu) is absorbed from the gastrointestinal tract, and enters the systemic circulation. It is then taken up by muscle, fat, and other tissues via the action of insulin secreted from the pancreas, and is used or stored as an energy source. Type-2 diabetes is caused by the retention of Glu in the blood due to the impaired action of insulin [13,14]. One approach to prevent diabetes is to improve people’s eating and exercise habits. The purpose of improving eating habits, for example, by eating slowly, avoiding overeating, and increasing intake of vegetables, is to suppress sudden rises in blood Glu levels [15].
Alginic acid (Alg) is a polysaccharide derived from brown algae, such as kelp and seaweed. Alg and its salts (calcium alginate (Ca- Alg), sodium alginate (Na-Alg), ammonium alginate, and potassium alginate) are non-toxic; their daily tolerable intake is described as "not specified" by the Ministry of Health, Labor and Welfare, Japan [16]. Na-Alg is used as a food additive (thickening stabilizer) and medicinal additive, and also as a health food and pharmaceutical, because it has a blood cholesterol-lowering effect and a protective effect on the gastric mucosa [17]. However, chronic high intake of sodium is among the risk factors for hypertension [18]. Therefore, Ca-Alg might be preferable to Na-Alg, if its cholesterol-lowering effect is equal to or greater than that of Na-Alg.
Ca-Alghas a wide range of beneficial biological effects. We have shown that Ca-Alg promotes the excretion of heavy metals such as strontium and cesium in rats, and we examined the mechanism involved [19,20]. We found that Ca-Alg inhibits the reabsorption of bile acids in the gastrointestinal tract and promotes their excretion into feces in rats, thereby promoting catabolism of cholesterol to bile acids in the liver and resulting in a decrease of blood cholesterol [21]. We also showed that Ca- Alg inhibits the absorption of triglyceride (lipid) from food by forming large micelles with triglyceride as their core [22].
It has also been suggested that Ca-Alg suppresses blood Glu elevation [23]. We were interested in this action, and investigated the effect of Ca-Alg in rats, focusing on the influence of the particle size of Ca-Alg and the amount of Ca- Alg added to the diet [24]. We subsequently carried out clinical trials in which healthy adults were asked to consume various foods, i.e., udon noodles [25], soba (buckwheat noodles) [26], and Chinese noodles [27] containing Ca-Alg, and we measured their changes of blood Glu levels. Here we review the results of these experimental studies and clinical trials, and summarize the effects of Ca-Alg on postprandial blood Glu elevation.
In the gastrointestinal tract, starch is first degraded to maltose, mainly by α-amylase, and then maltose is degraded to Glu by α-glucosidase. The resulting Glu is recognized by a specific transporter on the cell membrane surface, and transferred to the blood. Since Ca-Alg is hardly absorbed in the digestive tract, it must inhibit one of these processes in order to suppress postprandial blood Glu elevation.
In vitro studies showed that Ca-Alg concentration-dependently inhibits α-glucosidase activity, and the rate of inhibition was 38.2% when the Ca-Alg concentration was 3.53 mg/mL (Figure 1). α-Amylase was also inhibited, but the inhibition rate was only 2.1% even at the highest Ca-Alg concentration of 3.53 mg/mL. The amount of Glu adsorbed on Ca-Alg depended on the initial Glu concentration, and was saturated at high Glu concentration; the maximum adsorption amount Vmax was 10.8 (μmol /mg Na-Alg), and the binding constant Km was 155.3 (mM) [24]. These values suggest that only a half of Ca-Alg binds Glu at the Glu concentration of about 150 mM, indicating that the affinity between Glu and Ca-Alg is weak.
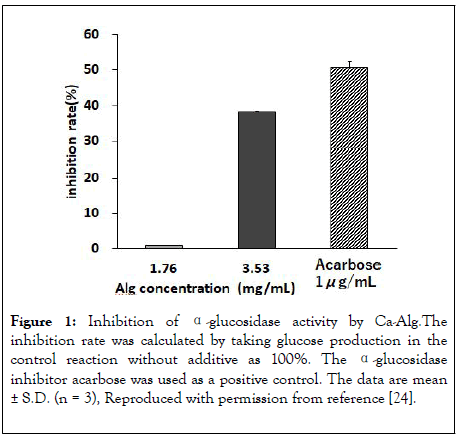
Figure 1: Inhibition of α-glucosidase activity by Ca-Alg.The inhibition rate was calculated by taking glucose production in the control reaction without additive as 100%. The α-glucosidase inhibitor acarbose was used as a positive control. The data are mean ± S.D. (n = 3), Reproduced with permission from reference [24].
We also found that Glu uptake by Caco-2 cells, a human colonderived cell, was unaffected by Ca-Alg, whereas it was significantly inhibited by the GLUT2 inhibitor phloretin, used as a positive control. These results suggest that Ca-Alg inhibits the metabolism of starch in the gastrointestinal tract by inhibiting the activity of α-glucosidase, which degrades maltose to Glu, resulting in reduced Glu absorption (Figure 2) [24].
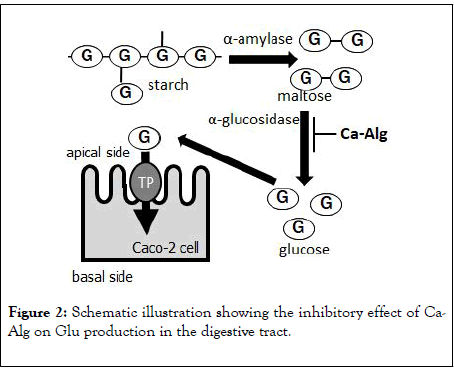
Figure 2: Schematic illustration showing the inhibitory effect of Ca- Alg on Glu production in the digestive tract.
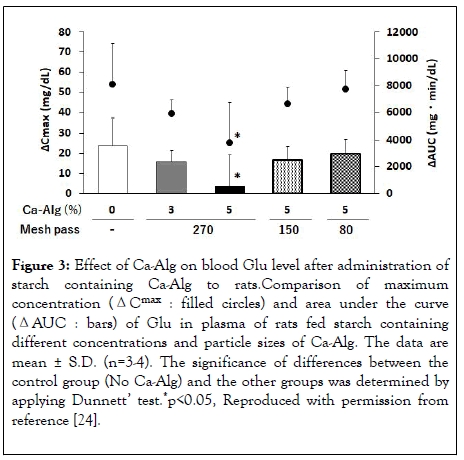
Figure 3: Effect of Ca-Alg on blood Glu level after administration of starch containing Ca-Alg to rats.Comparison of maximum concentration (ΔCmax : filled circles) and area under the curve (ΔAUC : bars) of Glu in plasma of rats fed starch containing different concentrations and particle sizes of Ca-Alg. The data are mean ± S.D. (n=3-4). The significance of differences between the control group (No Ca-Alg) and the other groups was determined by applying Dunnett’ test.*p<0.05, Reproduced with permission from reference [24].
We first examined the effects of Ca-Alg content and particle size in experimental animals. Rats were divided into three groups, which were orally administered starch containing no Ca-Alg, or containing 3 or 5% Ca-Alg with a particle size of 270 mesh pass. Blood samples were collected at intervals after feeding to measure blood Glu levels, and the difference between the preprandial and postprandial blood Glu levels was calculated at each time point (ΔC). The maximum value of ΔC (ΔCmax) was significantly reduced by 5% Ca-Alg, and the rapid postprandial rise in blood Glu level was suppressed. At the same time, there was a significant decrease in the area under ΔC-time curve (ΔAUC), indicating a decrease in total Glu absorption [24].
Next, the effect of the particle size of the Ca-Alg used was examined. Rats were divided into four groups and orally administered starch containing no Ca-Alg or 5% Ca-Alg with particle sizes of 80, 150 and 270 mesh pass. Blood samples were collected at intervals. The values of ΔCmax and ΔAUC were significantly reduced in the 270 mesh Ca-Alg-containing starchfed group. No effect was observed in the groups given starch containing 80 and 150 mesh pass Ca-Alg ( ure 3). These results suggest that the inhibitory effect of Ca-Alg on postprandial blood Glu elevation is dependent on the particle size of Ca-Alg. It seems likely that these results reflect the difference in surface area of Ca-Alg among the different particle sizes [24] Figure 3.
In these animal experiments, the drug was administered directly into the stomach, so the effect of mastication was not taken into account. In addition, there are species differences in the intestinal environment and enzyme activities, so it is difficult to extrapolate these results to humans. Therefore, we next examined whether similar effects could be obtained in clinical trials.
Udon, buckwheat and Chinese noodles account for more than 50% of all noodle sales in Japan [28]. However, in recent years, awareness of the high Glu contents of these noodles and their effects on blood Glu levels has been increasing concomitantly with a rise in health consciousness.
Udon noodles consist of long, relatively thin strands, which are sold in dry, boiled and raw forms. Udon has been eaten throughout Japan since the early Edo period (1603~1700 AD). In this trial, we asked healthy adults to consume udon containing various amounts of Ca-Alg (Figure 4).

Figure 4: Photos of udon, Japanese traditional noodles.
The trial design was a prospective, randomized, double-blind, three-group, three-phase crossover study in healthy Japanese adults. Blood Glu levels were monitored after subjects had ingested udon containing no, 5% or 8% Ca-Alg. Insulin (IRI), total cholesterol (T-Cho), high-density lipoprotein cholesterol (HDL-Cho), low-density lipoprotein cholesterol (LDL-Cho), triglyceride (TG), uric acid (UA), urea nitrogen (BUN) and Ca levels in blood were also investigated. The subjects were given a postprandial questionnaire focusing on texture and other features of the noodles [25].
Subjects who ate the control noodles showed the highest blood Glu levels at 30 to 60 minutes after ingestion. ΔCmax was 50 mg/dL, and the ΔAUC was 3365 mg min/dL. A significant decrease in ΔCmax was observed in the groups that ate the noodles containing 5% or 8% Ca-Alg compared to the control: ΔCmax was 45 mg/dL (change: -11%) for 5% Ca-Alg and 43 mg/dL (-15%) for 8% Ca-Alg. Furthermore, ΔAUC was 2847 mg・min/dL (-15%) for 5% Ca-Alg and 2650 mg・min/dL (-21%) for 8% Ca-Alg. Thus, the finding [24] that Ca-Alg has a blood Glu-suppressing effect in rats was reproduced in humans (Figure 5).
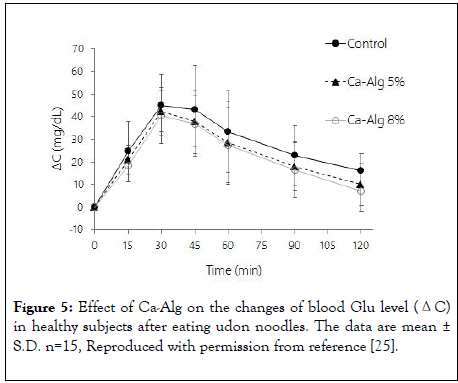
Figure 5: Effect of Ca-Alg on the changes of blood Glu level (ΔC) in healthy subjects after eating udon noodles. The data are mean ± S.D. n=15, Reproduced with permission from reference [25].
After subjects had eaten the noodles containing 5% or 8% Ca- Alg, their blood Ca concentration was increased significantly at 120 minutes. This suggests that Ca derived from Ca-Alg was absorbed into the body. The ΔT-Cho value showed a tendency to decrease in both Ca-Alg groups at 30 minutes after administration, and the ΔT-Cho value was decreased slightly but significantly in the 8% Ca-Alg group at 120 minutes. There were no significant changes in other blood test values. The postprandial questionnaire answers concerning the noodles' chewability, thickness, and taste preference revealed no significant difference in the subjects’ evaluation of the three types of noodles.
Soba is kind of Japanese noodle processed using powdered buckwheat, according to a recipe dating back to the late 16th century. Usually, flour is mixed in to make the noodles easier to cut.
The clinical trial with soba noodles was designed as a prospective, randomized, double-blind, crossover study. Healthy adult males and females aged 35 or older were randomly assigned to test or control diet groups after eligibility assessment, with consent. With reference to the amount of Ca-Alg determined to be optimal in the previous clinical study [25], noodles with 3.1 g (1.7% of product) of Ca-Alg per serving were used as test meals, and noodles containing no Alg were used as control meals. Postprandial blood Glu levels were monitored at intervals [26].
The blood Glu level of the control diet group showed a ΔCmax value of 58.3 mg/dL at 47.8 minutes (Tmax) after ingestion, and ΔAUC was 3888 mg·min/dL. The ΔCmax of the test meal group was 50.9 mg/dL at 40 minutes (Tmax) after ingestion, and ΔAUC was 3324 mg·min/dL. Compared with the control diet group, the ΔCmax of the test diet group was significantly reduced by -12.7%. The ΔAUC also decreased significantly, and the change was -14.5%. There was no significant difference in Tmax between the control diet and the test diet groups. These results suggest that Ca-Alg-containing soba noodles can suppress the increase in blood Glu level and decrease the total amount of absorbed Glu, similarly to Ca-Alg-containing udon noodles (Figure 6).
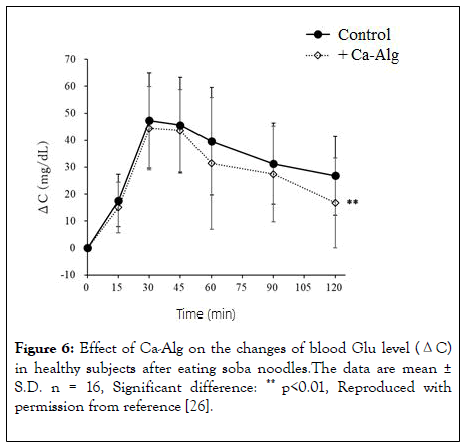
Figure 6: Effect of Ca-Alg on the changes of blood Glu level (ΔC) in healthy subjects after eating soba noodles.The data are mean ± S.D. n = 16, Significant difference: ** p<0.01, Reproduced with permission from reference [26].
In accordance with the previous finding [25], an increase in blood Ca concentration at 120 minutes after eating the test food was also observed in this trial, though in this case it was not statistically significant. The uric acid level at 120 minutes after the consumption of the test meal was also significantly lower than that in the control group, although the reason for this is not clear. In addition, an increasing tendency was observed in the change of Ca (ΔCa120min). There were no significant changes in other blood test values, and no significant differences between the control and the test diet groups regarding the evaluation of taste, texture, and bowel movements. Overall, the results are similar to those in the previous report [25], and indicate that Ca-Alg suppresses blood Glu level increase and reduces Glu absorption when added to soba noodles.
Chinese noodles were selected the third test product, and evaluated in a randomized, double-blind, crossover trial [27]. As in the previous trials, healthy adult males and females aged 35 years or older were given noodles supplemented with 3 g of Ca- Alg per serving as a test meal (1.9% of product), based on the amount of Ca-Alg determined to be optimal in the previous clinical trials [25,26]Ca-Alg-free noodles were used as a control food. Twenty-two healthy adult males and females aged 35 or older were randomly assigned to a test or control diet group. A questionnaire was conducted on the test day, and after the second test day, participants were asked about changes in their bowel movements.
From 45 minutes after ingestion, the blood Glu level of the test diet group was significantly lower than that of the control diet group. The blood Glu level of the control diet group showed ΔCmax of 50.3 mg/dL at 51 minutes (Tmax) after ingestion, and ΔAUC was 3724 mg·min/dL. The blood Glu level of the test meal group reached its maximum at 51 minutes (Tmax) after ingestion. ΔCmax was 45.5 mg/dL, and ΔAUC was 3123 mg·min/dL. Compared with the control diet group, the ΔCmax of the test diet group was significantly reduced by -9.5%. The ΔAUC was also decreased significantly (-16.1%). There was no significant difference in the Tmax between the control and the test diet groups. In addition, changes in insulin and Glu at 120 minutes after eating the test meal were also significantly reduced as compared with the control diet group (Figure 7). In addition, the concentration in Ca in blood was significantly increased. There was no significant difference between the control and the test diet group in the evaluation of taste, texture, or bowel movements.
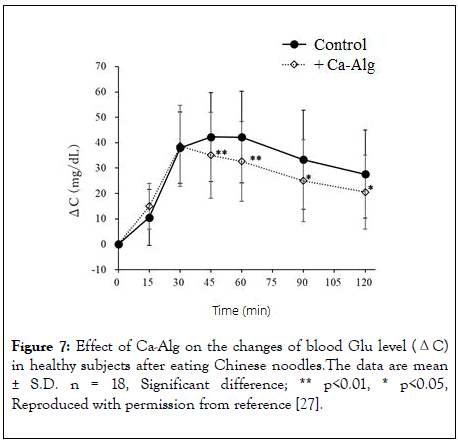
Figure 7: Effect of Ca-Alg on the changes of blood Glu level (ΔC) in healthy subjects after eating Chinese noodles.The data are mean ± S.D. n = 18, Significant difference; ** p<0.01, * p<0.05, Reproduced with permission from reference [27].
The test diet group showed a significant decrease in ΔCmax and ΔAUC compared with the control diet group. In particular, the Glu concentration at 120 minutes after eating the test food was significantly reduced. The results indicate that the inclusion of Ca-Alg suppressed the rise in blood Glu levels and decreased the total absorption of Glu. The significant increase in Ca and decrease in blood insulin concentration at 120 minutes after eating the test food are similar to those seen following the consumption of udon or buckwheat noodles [25,26]. Insulin is known to increase following a rise in blood Glu, and thus the suppression of blood Glu rise caused by Ca- Alg was presumably the reason for the decrease in insulin secretion [27-36].
As the Japanese population has become increasingly health consciousness, Ca-Alg-containing foods have been considered to be useful to suppress not only the sharp rise in postprandial blood Glu levels, but also the total absorption of Glu without the need for dieting. Our findings that Ca-Alg decreased blood Glu levels and suppressed the total absorption of Glu from all of the foods examined in the three trials (Table 1) are consistent with this belief. Moreover, when the Ca-Alg noodles were used as a test food, an increase in blood Ca concentration after the meal was observed. This suggests that Ca derived from Ca-Alg was absorbed into the body, so it is possible that incorporating Ca-Alg into the diet might help to prevent problems due to Ca deficiency, such as osteoporosis. Interestingly, this phenomenon has not been observed in animal experiments to date. In some cases, decreases of Cho, BUN, and uric acid levels in blood were observed, although further studies would be needed to confirm these changes and to establish the mechanisms involved.
| Contents of Ca-Ag | Glu level (% of control) | Other efects at 120 min | |||||
|---|---|---|---|---|---|---|---|
| Entry | % of product | % of Dried product | Δ C max | Δ AUC | Increasing in blood | Decreasing in blood | Reference |
| 1 | 1.8 | 4.6* | -11 | -15 | Δ Ca | [25] | |
| 2 | 2.8 | 7.3** | -18 | -21 | Δ Ca+ | Δ T-Cho | [25] |
| 3 | 1.7 | - | -12.7 | -14.5 | Δ Ca | Uric acid | [26] |
| 4 | 1.9 | - | -9.5 | -16.1 | Δ Ca | Insulin | [27] |
+: Not significant,*: Notation as 5% in literature, **:
Notation as 8% in literature
Table 1: Inhibitory effect of calcium alginate on increase in blood glucose level in clinical studies.
Various other substances, including indigestible dextrin, dietary fiber made from corn starch hydrolysate, salacinol from plant salasia, proanthocyanidin from acacia, and guar gum, can also slow down the absorption of Glu. For example, indigestible dextrins inhibit α-glucosidase, and have been widely used as foods for specified health use and functionally labeled foods. Our findings show that Ca-Alg also inhibits α-glucosidase, and its decreasing effect on blood Glu levels was greater than that of indigestible dextrin. Furthermore, the results of the questionnaire showed that incorporation of Ca-Alg into noodles had no significant effect on the acceptability of the noodles. Therefore, foods containing Ca-Alg are expected to be helpful to prevent the onset of diabetes without the need for individuals to alter their dietary preferences.
Kazuyo Shiragami and Nobuyuki Obara are employees of Shimadaya Corporation. Ogihara Takuo has no conflict of interest.
Citation: Shiragami K, Obara N, Ogihara T (2020) Inhibitory Effect of Calcium Alginate on Postprandial Blood Glucose Elevation: Basic and Clinical Studies. J Clin Trials 10:411. doi: 10.35248/2167-0870.20.10.411
Received: 22-Apr-2020 Accepted: 05-May-2020 Published: 12-May-2020 , DOI: 10.35248/2167-0870.20.10.411
Copyright: © 2020 Shiragami K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.