
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Case Report - (2023)Volume 14, Issue 3
Immunotherapy has revolutionized the treatment landscape for metastatic melanoma. This case report presents the clinical course of a patient with metastatic melanoma who received combination therapy with ipilimumab, a CTLA-4 checkpoint inhibitor, and an Indoleamine 2,3-Dioxygenase (IDO)-silenced Dendritic Cell (DC) vaccine. The treatment approach aimed to enhance antitumour immunity by blocking immune checkpoints and activating dendritic cells. The patient demonstrated a significant clinical response, indicating the diversification and potentiation of the antitumour effects. This case underscores the potential of combined immunotherapeutic strategies in achieving durable and robust antitumour effects.
Metastatic melanoma; Immunotherapy; Ipilimumab; Antitumour immunity
Metastatic melanoma is an aggressive form of skin cancer with limited treatment options and a high mortality rate. Immunotherapy, particularly immune checkpoint inhibitors, has emerged as a promising treatment strategy by harnessing the patient's immune system to recognize and attack cancer cells. This case highlights the use of combination therapy with ipilimumab, an anti-CTLA-4 monoclonal antibody, and an IDOsilenced dendritic cell vaccine in a patient with metastatic melanoma. The aim was to stimulate and diversify antitumour immune responses, potentially leading to improved outcomes [1].
Dendritic Cells (DCs) are the antigen-presenting cells that bridge the gap between innate and adaptive immunity [2]. DCs are being researched as vaccinations for cancer patients due to their powerful involvement in activating cytotoxic T cells. However, despite significant efforts to develop DC immunization as a cancer therapy, therapeutic effectiveness has been limited thus far. It has become clear that the presence of immune suppressive components in the immune system and tumor microenvironment is a major contributor to the dismal clinical outcomes [3].
The population of regulatory cells, which includes T-regulatory (Treg) cells, tolerogenic DCs and Myeloid-Derived Suppressor Cells (MDSCs), is one of the variables that contribute to immunosuppression. Treg and MDSC cells can connect with other cells like DCs to decrease their immune-activating activity. Tolerogenic DCs employ immunological inhibitory factors such as Interleukin-10 (IL-10), Indoleamine 2,3-Dioxygenase (IDO) and programmed death ligand-1 as one way. Immunosuppression caused by IL-10 is mediated via the impairment of DC maturation, T-cell and NK-cell activities [4,5]. IDO-positive DCs decrease T-cell responses and increase tolerance by depleting tryptophan and producing metabolic products, both of which can have an immunosuppressive impact. Furthermore, IDOpositive DCs drive T-cell development into Treg cells rather than clonal proliferation and effector cell differentiation [6].
A 45-year-old male presented with metastatic melanoma, involving multiple lymph nodes and distant organs. The patient's disease had progressed despite previous treatments, including surgery, radiation therapy, and chemotherapy. Based on the patient's eligibility and informed consent, combination immunotherapy was initiated, consisting of ipilimumab and an IDO-silenced dendritic cell vaccine (Figure 1).
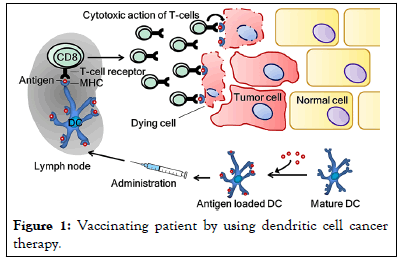
Figure 1: Vaccinating patient by using dendritic cell cancer therapy.
Clinical response
Following the treatment initiation, the patient experienced a notable clinical response. Progressive reduction in the size of metastatic lesions was observed, both locally and at distant sites, as assessed by imaging studies. The patient also reported a significant improvement in overall well-being, including reduced pain and fatigue.
Immune monitoring
Immune monitoring was performed to assess the diversification and potency of the antitumour immune response. Peripheral blood samples were collected at baseline and after treatment initiation. Flow cytometry analysis revealed an increase in the frequencies of activated T cells, including CD8+ cytotoxic T cells and memory CD4+ T cells. Additionally, the patient demonstrated a substantial increase in the production of proinflammatory cytokines, such as Interferon-γ (IFN-γ) and Tumor Necrosis Factor-α (TNF-α).
Safety profile
Adverse events related to treatment were monitored closely. The patient experienced manageable immune-related adverse events, including skin rash, fatigue and mild gastrointestinal symptoms. These adverse events were effectively managed with supportive care and temporary discontinuation of treatment.
The combination of ipilimumab and an IDO-silenced dendritic cell vaccine represents a rational approach to enhance antitumour immunity in metastatic melanoma. Ipilimumab blocks the CTLA-4 checkpoint, promoting T cell activation and proliferation. Concurrently, the IDO-silenced dendritic cell vaccine aims to overcome immunosuppression by targeting IDO, which is involved in the induction of immune tolerance [7].
The observed clinical response and immune monitoring results suggest the successful diversification and potentiation of the patient's antitumour immune response [8]. Increased frequencies of activated T cells and enhanced production of proinflammatory cytokines indicate the induction of a robust immune response against the melanoma cells.
The way that immunotherapies can diversify antitumor immunity is demonstrated by the activation of CD8+ T-cells against MART-1 and NY-ESO-1 in response to the vaccination and perhaps to ipilimumab [9]. Additionally, the production of a B-cell response against a number of putative melanoma antigens, some of which may be altered proteins (neoantigens), shows the existence of a continuous immune response, which might account for the patient's much reduced lung, liver and skin metastases.
Although immune identification of neoantigens has the ability to eradicate growing tumors, in this instance there has been therapeutic resistance. The genetic inactivation of the MHC class 1 2 microglobulin component, which is very common in numerous tumour types, including melanomas, is one of the suggested mechanisms that helps tumors evade the immune system onslaught. Therefore, the upregulation of tumourassociated antigens and/or the emergence of neoantigens will not inhibit tumor progression via TCR-activated T cells in patients with defective antigen presentation [10].
As a result, evaluating the functioning of antigen presentation pathways in tumor cells is necessary for a vaccination idea based on neoantigens [11]. IDO has also been suggested as a possible mechanism for the immunosuppression caused by melanoma. According to this theory, Allison and colleagues showed that IDO can reduce tumour-infiltrating effector T-cells while favoring the expansion of Treg cells, which can limit antitumor responses in the setting of ipilimumab treatment [12]. Therefore, combination therapies utilizing siRNA technology or 1-methyl-tryptophan, which are now in Phase I clinical studies, are urgently needed.
This case report highlights the clinical response and immunological changes observed in a patient with metastatic melanoma treated with combination therapy comprising ipilimumab and an IDO-silenced dendritic cell vaccine. The diversification and potentiation of the antitumour immune response demonstrated the potential efficacy of this therapeutic approach. Further research and larger clinical studies are warranted to validate these findings and optimize treatment strategies for metastatic melanoma.
The authors declare no competing interests.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Chen Q, Liu W, Zhoa Z (2023) Indoleamine 2,3-Dioxygenase (IDO); Silenced Dendritic Cell Vaccine with Ipilimumab in Treating Metastatic Melanoma Patients: A Case Report. J Clin Cell Immunol. 14:688.
Received: 15-May-2023, Manuscript No. JCCI-23-24583; Editor assigned: 17-May-2023, Pre QC No. JCCI-23-24583 (PQ); Reviewed: 31-May-2023, QC No. JCCI-23-24583; Revised: 07-Jun-2023, Manuscript No. JCCI-23-24583 (R); Published: 15-Jun-2023 , DOI: 10.35248/2155-9899.23.14.688
Copyright: © 2023 Chen Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.