Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Research Article - (2022)Volume 12, Issue 5
Following the breakthrough of Immune Checkpoint Inhibitors (ICIs), a new era of immuno-oncology agents has emerged and established immunotherapy as a part of cancer treatment. Despite the improving outcomes and durability of response to ICIs, unfortunately many patients with initial response are known to develop acquired resistance later. There is increasing interest in potentially using anti-pathogen immune response to induce an antitumor immune response, which has led to rethinking into how the efficacy of anti-pathogen could be further enhanced. The immunostimulatory effects of anti-pathogen treated tumors in combinations with ICI are known to potentially amplify anti-tumor immunity resulting in increased tumor responses and improved outcomes. Antipathogen treated tumors are immune-infiltrated “hot” tumors demonstrate higher treatment response rates and improved survival. Our research group has previously demonstrated that tumors can be converted from “cold” to “hot” by intratumoral injection of a commercially available seasonal influenza vaccine. In continuation with our work, in deciphering the role of anti-viral immunity in tumor immunology, we studied the role of inactivated SARSCoV- 2 virus as anti-tumor. Here we report that intratumoral injections of inactivated SARS-CoV-2 converts the immunologically cold tumors to hot tumors by generating anti-tumor mediated CD8 T cells. Our findings suggest that inactivated SARS-CoV-2 can be used as an immune modulator in immunotherapy in melanoma and triple negative breast cancer.
Immune checkpoint inhibitors; Immune microenvironment; Immunotherapy; Melanoma
The last decade has experienced a major addition to the cancer therapy arsenal. Antibody-based immunotherapies have been introduced to exploit the precision of the immune system to specifically target tumor cells and cancer driving immune cell populations. Thus, Immune Checkpoint Inhibitors (ICIs) have rapidly altered the treatment topography of multiple tumor types. Despite the characteristic durability of response to ICI, significant variability in effectiveness still exists between cancers, and even between individual patients with the same cancer type.
The heterogeneity in response to ICIs is due to multiple factors but may be related to differences in the composition of the Tumor Immune Micro Environment (TIME). TIMEs can be classified broadly into those that are devoid of immune cells, or those that are immune cell infiltrated. Further, the infiltrated TIMEs may contain different cell populations; some may be inflamed with pro-immune cytotoxic lymphocytes, while others are saturated with immunosuppressive cell populations. Previous studies have demonstrated that different TIMEs may result in different effects of immunotherapy. Checkpoint inhibitors produce greater antitumor effects when the TIME is inflamed, or “hot,” compared to tumors that are “cold,” or less immunogenic.
In December 2019, our group published a repurposing of the seasonal influenza vaccine as a cancer immune therapy [1], where intratumoral administration of the vaccine converted immunologically “cold” tumors to “hot”. Host response to pathogens enhanced antitumor immunity, notably thorough an increase in tumor-specific CD8+, T cells. Additionally, influenza vaccine treatment sensitized tumors to immune checkpoint inhibitor therapy. Notably, these effects were only seen after injection with the non-adjuvanted vaccine containing heatinactivated influenza virus, suggesting that some vaccines may be more immunogenic than others.
Our study on intratumoral injection of heat inactivated influenza virus as an immunotherapy agent was immediately followed by the onset of the COVID-19 pandemic. The rapid spread of SARSCoV- 2 established itself as a highly immunogenic virus, with notable similarities to influenza including both viruses are singlestranded RNA viruses [2]. Both viruses infect the respiratory tract and use surface proteins to infect the host. Influenza requires hemagglutinin [3] and neuraminidase for host cell invasion, whereas SARS-CoV-2 relies on protein S. Both viruses depend on a viral RNA polymerase to express their proteins. Given that several clinical cases have been reported where in COVID-19 infections were associated with tumor regression in melanoma [4] and hodgkin lymphoma [5,6], we hypothesized that a host response to intratumoral injection of chemically inactivated SARS-CoV-2 may produce an anti-tumor effect in mouse models akin to what we observed with influenza. In this study our findings propose that inactivated SARS-CoV-2 vaccines may have the potential for use as a cancer immunotherapy.
Mice
BALB/C and B6 (C57BL/6J) mice were purchased from The Jackson Laboratory at 6-8 weeks of age. All animals were housed in specific-pathogen-free facilities and all experimental procedures were in accordance with policies approved by the Institutional Animal Care and Use Committee (IACUC) and Rush University Medical Center.
Inactivated SARS-CoV-2 virus
Chemically inactivated COVID-19 was purchased from Microbiologics San Diego. For TLR-activation assays, 1.5 × 106 Plaque-Forming Units (PFU) per mL of inactivated COVID-19 was used. For all animal experiments, mice were administered 50 μl of inactivated COVID at a concentration of 1 × 106 PFU/ mL via intratumoral injection. Control mice received the same volume of Phosphate Buffer Saline (PBS) via the same route.
Seasonal influenza vaccine
Fluarix Quadrivalent (GlaxoSmithKline), a 2020-2021 FDA approved seasonal influenza vaccines was purchased for these studies: 50 μl of influenza vaccine or a PBS control were administered to mice via intratumoral injection. For Toll-Like Receptors (TLR) activation assays, Fluarix was diluted 1:2 in PBS prior to prior to plating.
Cell culture
HEK-Blue TLR reporter cell lines (Invivogen) were transfected with plasmids containing a murine TLR and an NF-κB inducible Secreted Embryonic Alkaline Phosphatase (SEAP) reporter gene, used to determine TLR stimulation. mTLR7, and non-TLR expressing parental cell line, Null2k, (Invivogen) were cultured using Dulbecco's Modified Eagle Medium (DMEM) (Gibco), supplemented with 10% Fetal Bovine Serum (Corning), 100 units/mL penicillin (Gibco), 100 mg/mL streptomycin (Gibco), and 100 μg/mL normocin (Invivogen). Expression of TLR and SEAP plasmids were maintained with the addition of selective antibiotics blasticidin (30 μg/mL, Invivogen) and zeocin (100 μg/mL, Invivogen). Triple-negative breast cancer cell line 4T1 was cultured in RPMI (Gibco), supplemented with 10% Fetal Bovine Serum, 100 units/mL penicillin (Gibco), 100 mg/mL streptomycin (Gibco), and 100 μg/mL normocin (Invivogen). Murine melanoma cell line B16-F10 was cultured in DMEM supplemented with 10% FBS, 100 units/mL penicillin, 100 mg/ mL streptomycin, and 100 μg/mL normocin.
TLR activation assay
To evaluate TLR7 stimulation, 20 μl of inactivated COVID-19 or influenza vaccine was plated in duplicate or triplicate in 96-well plates. TLR7 agonist (CL264, 50 μg/mL) was run simultaneously as a positive control. 1X PBS was used as a negative control. After plating the treatments, 50,000 mTLR7 or Null-2k cells suspended in 180 μl HEK-Blue Detection (Invivogen) medium were added to the respective wells. All cells were incubated for 24 hours at 37°C and 5% CO2. Following incubation, TLR stimulation was quantified using a Cytation 3 plate reader (BioTek) measuring absorbance at 620 nm.
Tumor challenge
BALB/C mice were anesthetized with isoflurane and injected with 500,000 4T1 triple-negative breast cancer cells in 100 μl PBS in the Mammary Fat Pad (MFP). B6 mice were anesthetized with isoflurane and challenged with 100,000 B16-F10 melanoma cells in 100 μl PBS via intradermal injection. Tumor development was monitored using Vernier calipers, where tumor area was determined by two measurements in perpendicular directions. To comply with Institutional Animal Care and Use Committee (IACUC) policies, tumor bearing mice were humanely sacrificed upon tumor measurements reaching 15 mm in either direction.
Flow cytometry
Tumor bearing mice were humanely sacrificed via carbon dioxide inhalation. For IL-10 staining, mice were administered (intravenously, via retro-orbital injection) 50 μg of monensin (Sigma-Aldrich) dissolved in a diluted ethanol solution six hours prior to sacrifice. Tissues were processed as previously described (insert flu shot paper reference number here). Extracellular staining for flow cytometry was performed with antibodies targeting CD3, CD4, CD8, CD11b, CD11c, CD20, CD45, Ly-6G/Gr-1, and MHCII. All antibodies were purchased from BioLegend, BD, eBioscience, or R and D Systems. An extracellular stain cocktail comprised 1-5 μl/test combined with 0.25 μl/test of Live/Dead fixable aqua dead cell stain kit (405 nm excitation) (Invitrogen) in a total volume of 100 μl (made in PBS). The extracellular stain cocktail was added to each sample and subsequently incubated in a dark environment at room temperature for 30 minutes. Following extracellular staining, samples were washed twice with 200 μl PBS. For optimal intracellular staining, samples were permeabilized and fixed with 100 μl of Cytofix/Cytoperm [1] for 15 minutes at 4°C. Samples were subsequently washed with 100 μl of 1X Perm/Wash Buffer [1]. IL-10 targeting antibody (Bio-legend) was used at 1 μl/test in a total volume of 100 μl (made in 1X Perm/Wash Buffer). Samples were incubated with intracellular stain for 30 minutes at 4°C, protected from light. Samples were subsequently washed with 200 μl of 1X Perm/Wash Buffer twice, followed by two 200 μl PBS washes. Flow cytometry was performed on the BD LSRFortessa. Flow cytometry analysis was completed using FlowJo (BD, version 10).
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 9.1.2). For studies involving more than two groups, a 1-way ANOVA with Tukey correction was used to determine statistical significance. A two-way ANOVA was performed to determine statistical significance for studies with multiple time points. For all studies, a p-value <0.05 was considered statistically significant.
Intratumoral injection of inactivated SARS-CoV-2 reduces tumor growth in murine melanoma and triple negative breast cancer models
We previously reported that the growth of B16 melanoma tumors could be halted by intratumoral injection of an inactivated influenza vaccine, while treatment with live influenza virus did not alter tumor growth [1]. Using the B16 murine model, we compared the growth patterns of melanoma tumors after injecting either inactivated influenza-encompassed within a seasonal influenza vaccine (FluVx), inactivated SARS-CoV-2 (iCOVID), and buffer controls. SARS-CoV-2 caused tumor growth inhibition like that of influenza, both of which significantly halted growth compared to controls, as shown in Figure 1A. We then asked whether the effects of inactivated viruses were unique to melanoma, or if tumor growth may be affected in other cancer types. We elected to study triple negative breast cancer, as breast cancer would be ideal for clinical translation given that tumors are typically superficial and could be easily accessed for intratumoral treatment in humans. After establishing tumors in mice using the 4T1 triple negative breast cancer (TNBC) model, we repeated the same protocol by injecting FluVx, iCOVID, and negative control. FluVx and iCOVID were both observed to dramatically reduce tumor growth compared to control; tumors in the FluVx or iCOVID treated groups were less than half the size (quantify) of those in the control group as shown in Figures 1B-1D. These findings suggest that introduction of iCOVID in the tumor can elicit antitumor responses similar to that of FluVx, (Figure 1).
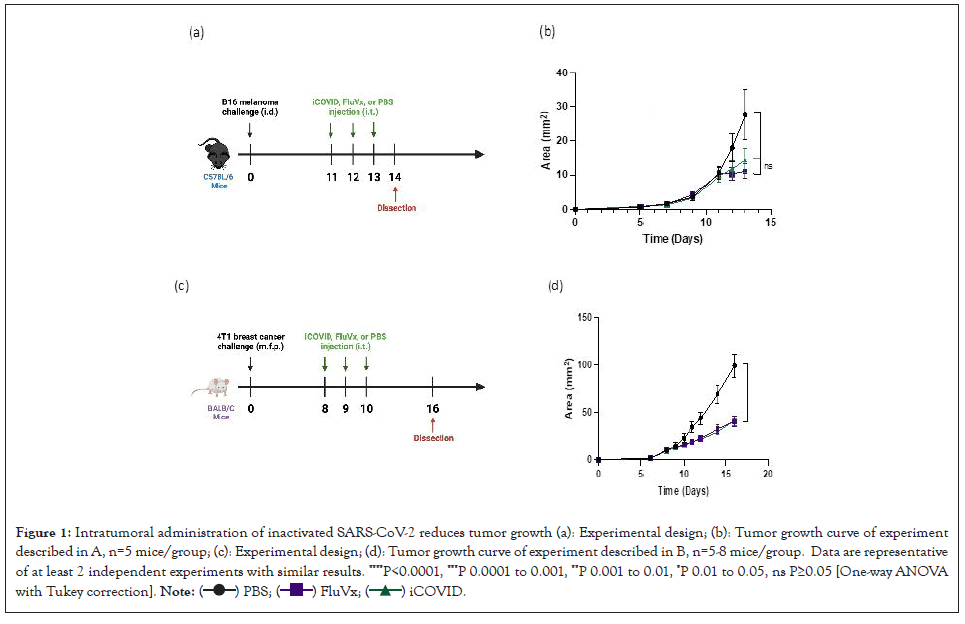
Figure 1: Intratumoral administration of inactivated SARS-CoV-2 reduces tumor growth (a): Experimental design; (b): Tumor growth curve of experiment
described in A, n=5 mice/group; (c): Experimental design; (d): Tumor growth curve of experiment described in B, n=5-8 mice/group. Data are representative
of at least 2 independent experiments with similar results. ****P<0.0001, ***P 0.0001 to 0.001, **P 0.001 to 0.01, *P 0.01 to 0.05, ns P≥0.05 [One-way ANOVA
with Tukey correction].
Reprogramming the tumor microenvironment with inactivated SARS-CoV-2 increases cross-presenting Dendritic Cells (DCs) and tumor antigen-specific CD8+ T cells
To determine the changes in the TIME following intratumoral administration of inactivated viruses in melanoma and breast cancer tumors, we performed flow cytometry analysis on treated tumor samples. Given that tumors with increased immune cell infiltration are associated with better response to immunotherapy and improved patient outcomes, we initially determined the amount of CD45+ within the tumor following treatment. In the B16 melanoma model there was a significant increase in CD45+ cell infiltration from FluVx and iCOVID treatment, however, there was no observed difference in CD45+ cell infiltration within the 4T1 tumor amongst the three groups as shown in Figures 2A and 2B. Because of the differences in immune cell infiltration between the two tumors models, we then assessed the changes in specific immune cell populations following each treatment. CD8 × T-cell infiltration in the tumor has been strongly associated with improved tumor outcomes, thus we were particularly interested in the effects of intratumoral iCOVID on T-cell infiltration especially when compared to FluVx. In melanoma and breast cancer models, intratumoral injection of iCOVID led to a significant increase in tumor infiltration of CD8+ T-cells compared to controls, like that seen following influenza injection as shown in Figures 2C and 2D. In addition to increased CD8+ T-cell infiltration following iCOVID and FluVx administration, both treatments were observed to increase Antigen Presenting Cell (APC) populations within the tumor. In the 4T1 tumor model a significant increase in CD8+ cross-presenting dendritic cells was observed in both FluVx and iCOVID treated tumors compared to control as shown in Figure 3A [7]. B16 melanoma tumors were found to have a significant increase in MHC-II+ cells in FluVx and iCOVID treated tumors as shown in Figure 3B. These findings suggest that intratumoral iCOVID can shift the TIME in a manner that is similar to that of FluVx (Figures 2 and 3).
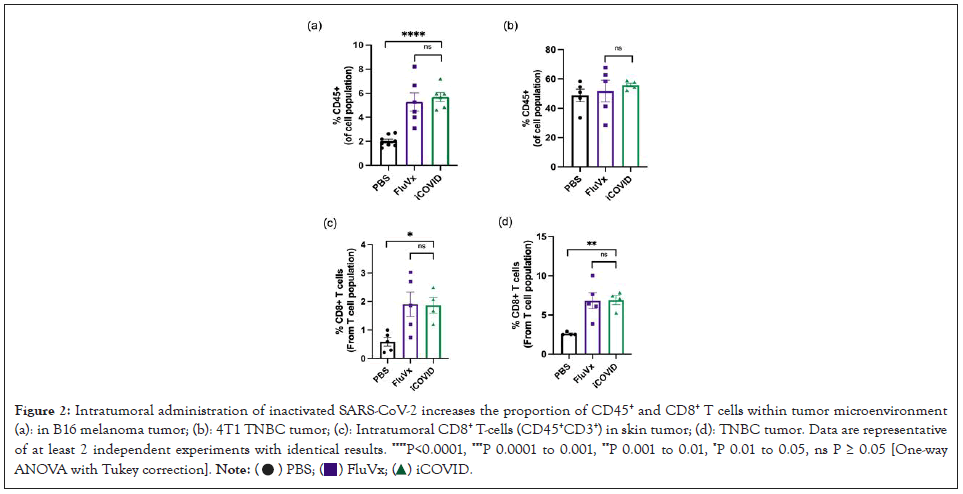
Figure 2: Intratumoral administration of inactivated SARS-CoV-2 increases the proportion of CD45+ and CD8+ T cells within tumor microenvironment (a): in B16 melanoma tumor; (b): 4T1 TNBC tumor; (c): Intratumoral CD8+ T-cells (CD45+CD3+) in skin tumor; (d): TNBC tumor. Data are representative
of at least 2 independent experiments with identical results. ****P<0.0001, ***P 0.0001 to 0.001, **P 0.001 to 0.01, *P 0.01 to 0.05, ns P ≥ 0.05 [One-way
ANOVA with Tukey correction].
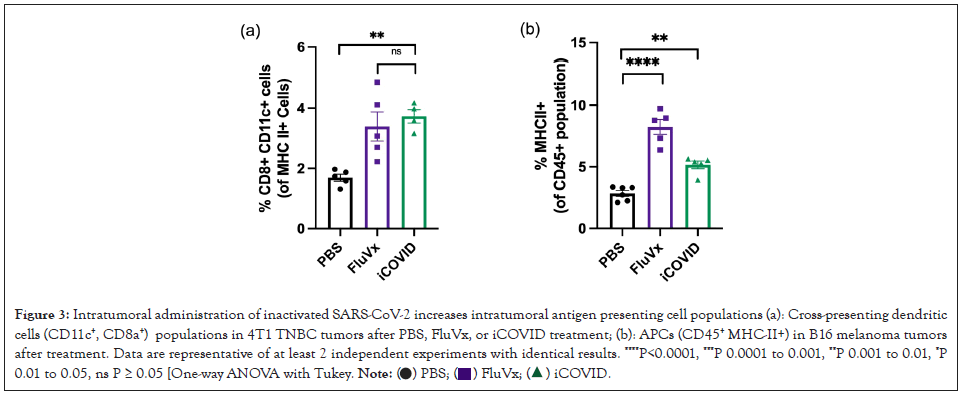
Figure 3: Intratumoral administration of inactivated SARS-CoV-2 increases intratumoral antigen presenting cell populations (a): Cross-presenting dendritic
cells (CD11c+, CD8a+) populations in 4T1 TNBC tumors after PBS, FluVx, or iCOVID treatment; (b): APCs (CD45+ MHC-II+) in B16 melanoma tumors
after treatment. Data are representative of at least 2 independent experiments with identical results. ****P<0.0001, ***P 0.0001 to 0.001, **P 0.001 to 0.01, *P
0.01 to 0.05, ns P ≥ 0.05 [One-way ANOVA with Tukey.
Inactivated SARS-CoV-2 treatment reduces myeloid- derived suppressor cell expansion in tumor microenvironment
Myeloid-Derived Suppressor Cells (MDSCs) are known to suppress T-cell response in the setting of cancer, thus promoting an immunosuppressive tumor microenvironment. Though not statistically significant, FluVx and iCOVID treatments both were observed to decrease CD11b+ Ly6G/Gr-1+ MDSC populations within 4T1 TNBC tumors as shown in Figure 4A. A similar trend was observed in B16 melanoma tumors. Intratumoral FluVx and iCOVID administration both lead to a significant decrease in MDSCs control in melanoma tumors as shown in Figure 4B. These findings suggest that inactivated SARS-CoV-2 and influenza are both capable of relieving the tumor microenvironment of immunosuppressive MDSC populations (Figure 4).
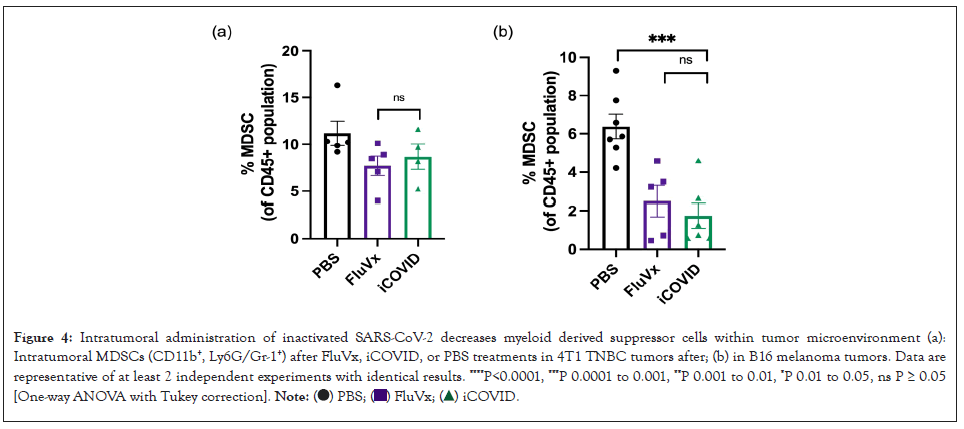
Figure 4: Intratumoral administration of inactivated SARS-CoV-2 decreases myeloid derived suppressor cells within tumor microenvironment (a):
Intratumoral MDSCs (CD11b+, Ly6G/Gr-1+) after FluVx, iCOVID, or PBS treatments in 4T1 TNBC tumors after; (b) in B16 melanoma tumors. Data are
representative of at least 2 independent experiments with identical results. ****P<0.0001, ***P 0.0001 to 0.001, **P 0.001 to 0.01, *P 0.01 to 0.05, ns P ≥ 0.05
[One-way ANOVA with Tukey correction].
SARS-CoV-2 induced tumor regression is TLR7- dependent
TLRs identify all microbial components, implying that TLRs function as adjuvant receptors to regulate both innate and adaptive immune responses [8]. It's possible that certain viral products act as TLR ligands and activate TLR7 signaling directly. Alternatively, like in the case of Drosophila host defense, viral infection may result in the production of endogenous ligandn [9]. TLR7 activity was found to be increased in response to single- stranded RNA (ssRNA), a natural agonist found in the SARS- CoV-2 as shown in Figure 5A, the data was comparable to what we discovered using FluVx1 as shown in Figure 5B (Figure 5).
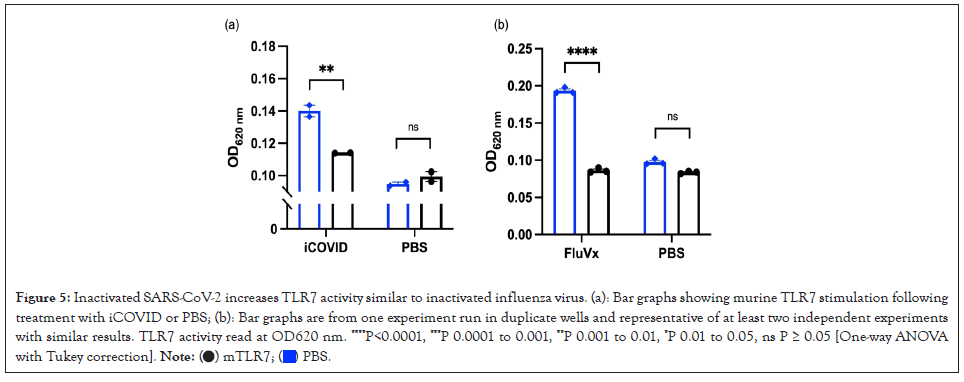
Figure 5: Inactivated SARS-CoV-2 increases TLR7 activity similar to inactivated influenza virus. (a): Bar graphs showing murine TLR7 stimulation following
treatment with iCOVID or PBS; (b): Bar graphs are from one experiment run in duplicate wells and representative of at least two independent experiments
with similar results. TLR7 activity read at OD620 nm. ****P<0.0001, ***P 0.0001 to 0.001, **P 0.001 to 0.01, *P 0.01 to 0.05, ns P ≥ 0.05 [One-way ANOVA
with Tukey correction].
Clinical breakthroughs using immunotherapy to improve and prolong the lives of cancer patients and have proved the immune system's importance in cancer treatment. However, immunotherapies have only been able to induce long-lasting responses in a small percentage of patients so far. As a result, new ways of engaging the immune system are required to take the next big step forward. Multiple lines of evidence suggest that local instigation of infectious microorganisms or synthetic molecules mimicking their components, the so-called Pathogen-Associated Molecular Patterns (PAMPs) acting as Pattern Recognition Receptor (PRRs) agonists, can be beneficial against cancers [10], ranging from Coley's clinical experiments with local treatment with bacteria or bacteria toxins to the generalized use of Bacillus of Calmette and Guerin (BCG) for superficial bladder cancer.
Multiple attempts have been made to target cytopathic effects against cancer utilizing so called oncolytic viruses in the case of viruses. A growing body of research suggests that replicationcompetent oncolytic viruses and replication-defective viral vectors work primarily by eliciting more effective antitumor immune responses. When the virus's genome contains genes that boost immunity, such as cytokines and costimulatory factors, the virus's potency can be increased. Intratumoral administration of viruses and virus-associated molecular patterns can result in anticancer effects, which are mostly mediated by the elicitation or potentiation of immune responses against the cancer.
According to recent findings in mice transplantable tumor models, the rotavirus, influenza, and yellow fever vaccinations are particularly well suited to eliciting potent antitumor immunity against cancer after intratumoral injection [11]. Adenovirus, vaccinia virus, Herpes Simplex 1 Virus (HSV), Newcastle disease virus, and reovirus intratumoral modified variations have showed exceptional activity in preclinical models and have moved or are advancing to the clinic [12]. T-Vec (Talimogene laherparepvec) [13], an attenuated HSV engineered to encode Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF), is approved for intratumoral infection in advanced melanoma4 and appears to have synergistic effects when combined with systemic treatment with anti-Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) or anti-programmed death-ligand. In our research, we used SARS-CoV-2 to boost naturally weak antitumor immune responses, resulting in better cancer outcomes, in case of triple negative breast cancer and skin cancer. Our findings show that inactivation of a nononcolytic virus, such as SARS-CoV-2 can boost an anti-cancer immune response when supplied via intratumoral injection. This shows that the field of microbialbased cancer treatments (MBCTs), which has recently regained popularity, is not restricted to oncolytic pathogens or even the use of active pathogens [14,15]. When homologous antigens are introduced within the tumor to induce an immune-"alarming" effect, virus-specific memory T cells have been proven to inhibit tumor growth in mice models [16].
Our findings suggest that injecting inactivated SARSCoV- 2 intratumorally reduces tumor growth by converting immunologically inactive cold tumors into immune-infiltrated hot tumors, thereby doubling the frequency of Dendritic Cells (DC) (including cross-presenting DCs) and tumor antigenspecific CD8+ T cells in the tumor microenvironment.
Citation: Giurini EF, Williams M, Morin A, Zloza A, Gupta KH (2022) Inactivated SARS-CoV-2 Reprograms the Tumor Immune Microenvironment and Elicits Anti-Tumor Response in Melanoma and Triple Negative Breast Cancer Murine Model. Trans Med. 12:268.
Received: 23-Aug-2022, Manuscript No. TMCR-22-18987; Editor assigned: 25-Aug-2022, Pre QC No. TMCR-22-18987 (PQ); Reviewed: 08-Sep-2022, QC No. TMCR-22-18987; Revised: 15-Sep-2022, Manuscript No. TMCR-22-18987 (R); Published: 26-Sep-2022 , DOI: 10.35248/2161-1025.22.12.268
Copyright: © 2022 Giurini EF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.