
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2021)Volume 11, Issue 4
Objective: Annually, billions of dollars are spent worldwide on drug development and the associated clinical trials conducted. Therefore, it is vital that pharmaceutical companies who sponsor clinical trials ensure that their data is accurate and timely. Numerous challenges for clinical trials exist and include recruitment and enrollment of appropriate research subject. Potential candidates for these studies are recruited and incentivized to participate in trials. While a great number of people participate in clinical trials solely for altruistic reasons, compensation for time and travel does motivate many potential research subjects. For others without adequate health insurance, the impetus is the evaluation and treatment with investigational products at no charge for their own potential health conditions. For both safety reasons and purposes of data integrity, it has long been held that research subjects should not volunteer in more than one study at a time. Also, there is typically a minimum 30-day waiting period or “washout period” between studies. These criteria are difficult to verify and thus we explored the development of a global regulatory compliant database that collects information on the exact research subject’s study history to detect multiple potential pitfalls and protocol violations that would be of immeasurable benefit to strengthen clinical trial data. Our study shows that subjects are neither always compliant nor forthcoming. There are attempts to screen more than once; there are age violations, washout period violations, and other violations that might cause poor quality data in a trial. Verified Clinical Trials (VCT) is the world’s largest and most comprehensive research subject database. By utilizing VCT, a sponsor can ensure that their subjects are verified and are not either enrolled in another clinical trial, still in their washout period, or in violation of any other protocol criterion.
Professional research subject; Duplicate subject; Clinical trial; Safety; Research subject database; Registry
One considerable challenge for clinical trials is the recruitment of appropriate and good-quality research subject candidates and their expedited enrollment in studies [1]. In order to detect multiple potential pitfalls and protocol violations, a global Health Insurance Portability and Accountability Act of 1996 (HIPAA) and General Data Protection Regulation (GDPR) compliant database that collects information on the specific research subject’s study history would be of immeasurable benefit to strengthen clinical trial data. The idea of having a centralized database is something that researchers have seen as a resolution to this issue, saying that “… the best protection against professional subject enrollment may be widespread adoption of a centralized subject registry” [1]. A registry will allow for less trial failure while saving money and time by eliminating unsuitable subjects from trials. This study explores the utility of a global research subject database registry and details the Verified Clinical Trials (VCT) database experience, including metrics that demonstrate real world evidence. For both safety reasons and purposes of data integrity, it has long been held that research subjects should not volunteer in more than one study at a time.
Typically, a minimum of 30-day waiting period or “washout period” is required between studies. Almost every clinical trial that involves Investigational Products (IP) has this requirement written into the inclusion and exclusion criteria of the study protocol. Unfortunately, a common occurrence in trials is the duplicate subject or “professional research subject.” These individuals seek out clinical trials in an effort to monetize their time by screening and enrolling in various clinical trials contemporaneously. In these instances when individuals join a study for nefarious reasons, the research subjects oftentimes will not adhere to dosing and may even not expose themselves to the IP. They may falsely tell the study coordinator that they have been compliant and provide fallacious answers to make it seem as if they are genuine. In these instances, this will indeed skew the placebo rates and make the IP appear ineffective. If there are just enough of these poor-quality subjects in a trial, it can result in failure to meet the primary endpoints and failure of a multimillion- dollar study. In some cases, the study may need to be repeated. In other cases, such as with smaller biotech companies, the program may be cancelled due to lack of confidence in the study drug or due to insufficient funds to repeat the study. Moreover, it is not only the duplicate subject violation that results in poor quality data. With so many competing studies, sponsors are concerned, and rightfully so, that subjects who are involved in multiple studies per year provide fake health conditions and untruthful answers to enroll in a study. Investigators are worried because by using these subjects make the study vulnerable to inaccuracies. Commonly, research volunteers who screen for a depression trial or pain study (among other health conditions) also screen for a healthy phase I study simultaneously. Research subjects do not always “stay in their lane” and very often will screen for a multitude of clinical trials across various therapeutic indications, even when they may not be affected by the medical condition of interest in the trial.
VCT (Garden City, NY, USA) is the world’s largest and most comprehensive research subject database. VCT is a global HIPAA and GDPR compliant network that monitors a research subject’s clinical trial participation and research study history via multi-point registration. VCT is employed extensively in phase I trials as well as in multi-site phase II through phase IV studies across numerous sites, early-phase units, and by pharmaceutical sponsors. The VCT system is utilized across numerous therapeutic indications. This review will elaborate on the process that protects the integrity of pharmaceutical study data by outlining the most common alerts. In this particular paper, we focus on an aggregated collection of a few study programs over a short period of time across psychiatric (central nervous system; CNS) indications that include Schizophrenia, Major Depressive Disorder (MDD), and Bipolar Depression (BPD). To ensure confidentiality, the pharmaceutical companies are labeled as “Sponsor” and the collection of their study programs will be noted as “Program”. We first analyzed the data in the program to illustrate the foundation of what VCT can perform. Next, we provided an example of the data analysis that VCT can provide by using important information to mitigate the risks posed by “professional” subjects. By examining the data and the subsequent analysis, we explain how VCT enriches and protects clinical trials, improving results, reducing placebo rates, saving money, and ultimately bolstering the success of the clinical trials.
The studies involved in this analysis span the last few years. At the time of writing, some studies have been completed while some are still ongoing. These sample trials are large trials with upwards of 30,000 verifications across this aggregated program. The verification flags that occurred throughout the screening process are presented and the top three exclusionary alerts overall were analyzed.
The VCT database utilizes multiple points of a research subject’s demographic information (along with optional fingerprint and optional facial biometric capabilities) to create a Unique proprietary Identification Code (UIC) that keeps the research subject’s Personal Identifying Information (PII) anonymized. Following the execution of the specific VCT Informed Consent Form (ICF), portions of the research participant’s name, Date of Birth (DOB), and sex are entered into the VCT system after confirmation from valid photo identification. Biometrics is optional and is utilized to improve speed and accuracy of verification. A staff member at the research site enters this information into the VCT portal at the time of screening. Ultimately, this UIC is used to verify each research subject’s eligibility into a specific clinical trial protocol. In just seconds, a result is displayed indicating whether the research subject is participating in another clinical trial concurrently or in violation of a protocol criterion based on past study history and participation. The entire verification process takes under three minutes. These protections continue throughout the entire duration of the study. Should a research subject attempt to screen or enroll in another study following verification in VCT, the subject will be prevented from doing enrolling in the second study. This ultimately improves retention within the study, as subjects will not be able to vanish from one study to enroll in another and skew sponsors’ data.
Data from the studies selected provides a comprehensive overview on CNS studies over short periods of time. The metrics reported include the different types of potential protocol violations and their relative importance, along with a breakdown of the age and gender of subjects and how that can impact data. Next, data was analyzed from all the sponsor’s trials in the VCT database to illustrate how many subjects are verified twice or more, then how subjects move between studies. The purpose is to demonstrate that subjects can and will violate protocol criteria and have tremendous capability to compromise a clinical trial if the sponsor does not take measures to ensure otherwise.
Figures 1 and 2 shows the different protocol violations aggregated from the different studies that were collected, that were reported. This is especially important because it clearly outlines that certain type of violations are more common than others, which will prove that VCT is vitally important, not just for the initial purpose of violation prevention, but also for the analysis of what the critical violations are, and how frequently they occur.
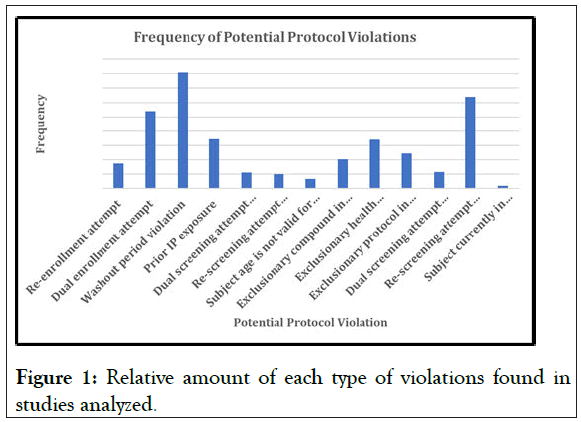
Figure 1: Relative amount of each type of violations found in studies analyzed.
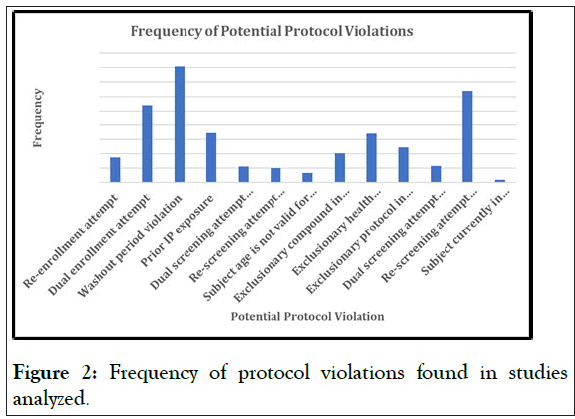
Figure 2: Frequency of protocol violations found in studies analyzed.
For example, the number of violations is not insignificant, and these are all criteria that are challenging for the site to verify during of violations is not insignificant, and these are all criteria that are challenging for the site to verify during the screening process, as the subject could choose to not disclose prior study history. Using the VCT, a simple, three-minute verification process, all the subject’s prior studies is available for the site to review against protocol criteria to ensure that the subject is eligible.
Figure 3 defines the number of subjects that have been verified multiple times, and these subjects are from only the collection of studies that were chosen to represent Phase II and III CNS studies, which clearly shows that multiple subjects are not doing only one study and then returning to their standard of care. Subjects move from study to study, whether due to a need for healthcare that is otherwise unavailable, or for financial reasons. Either way, these are not subjects that will provide good data, as they tend to be professional subjects, and those are the subjects that VCT can help elucidate in real time and assist the sponsor to prevent these subjects from entering trials. While not all these subjects were necessarily in violation, this graph serves purely to illustrate that there are subjects who will move from study to study, which causes the high number of washout period violations that were seen in the initial three graphs.
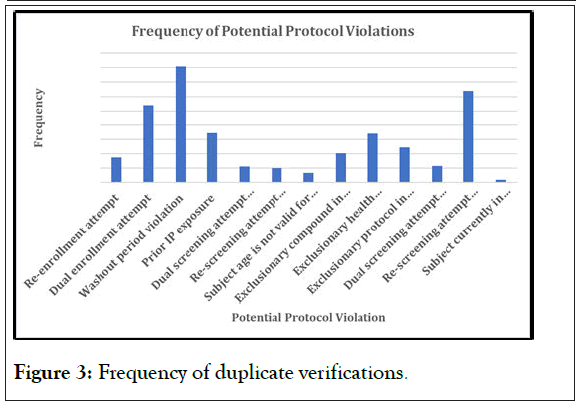
Figure 3: Frequency of duplicate verifications.
In Figures 4 and 5 (5A-5C), the verifications in the chosen studies are analyzed by age and by gender. At the first glance, we can see the equal distribution in both variables. The gender pie chart is almost perfectly even, and although there is a significant amount centered in the 40-60-year-old age range, there is data from the lower limit of 18 to the upper limit of 75 (18-75 is the most common protocol-defined age range, and this is what we would expect to see in any study analysis). This analysis is a perfect representation of the VCT database, which is expansive and diverse, with representation from all subject demographic parameters. A thorough and diverse database will exponentially benefit from additional trials, as it will add more subjects to the pool of data.
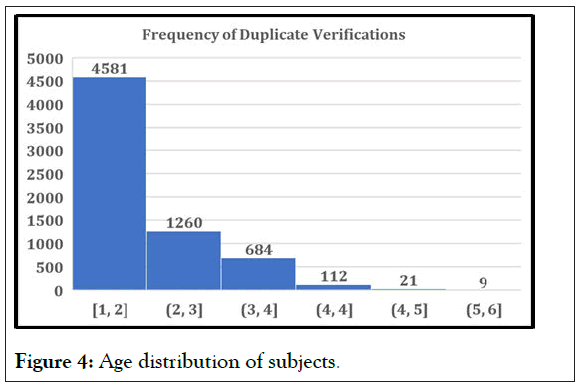
Figure 4: Age distribution of subjects.
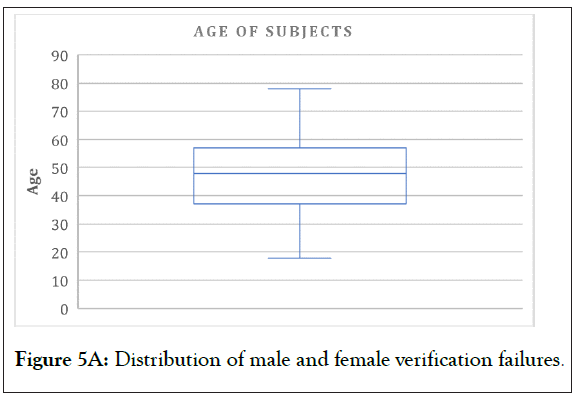
Figure 5A: Distribution of male and female verification failures.
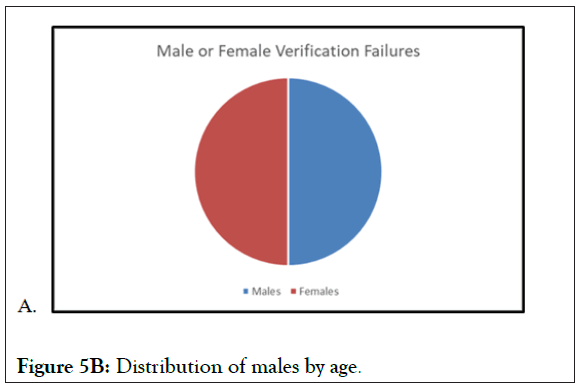
Figure 5B: Distribution of males by age.
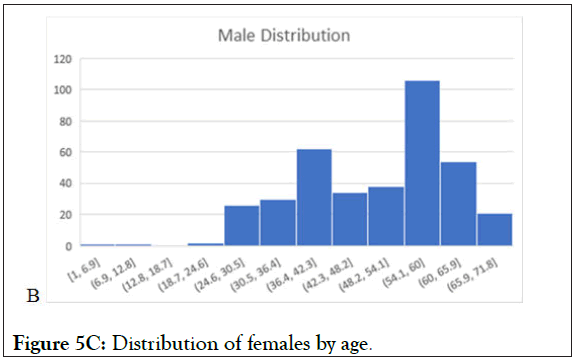
Figure 5C: Distribution of females by age.
Figure 6 (6A-6C) above represent the data, utilizing the verifications from every study analyzed with the VCT database. With the “True” indicative of yes, there is significant crossover by subjects between studies. As seen in prior graphs, this illustrates that subjects enroll in study to study, and subjects change between multiple conditions. One of the significant values of a research subject database is to detect and prevent subjects that are moving from study to study and condition to condition. Without a research subjects database registry such as VCT protection, the data from these trials is potentially irreparably flawed, greatly damaging the potential for IP approval and the finances of the sponsor, who may not be able to fund additional trials to recoup losses of data. If an FDA investigation finds instances of ineligible subjects receiving IP in the trial data, it compromises the potential IP approval and reflects poorly on the sponsor.
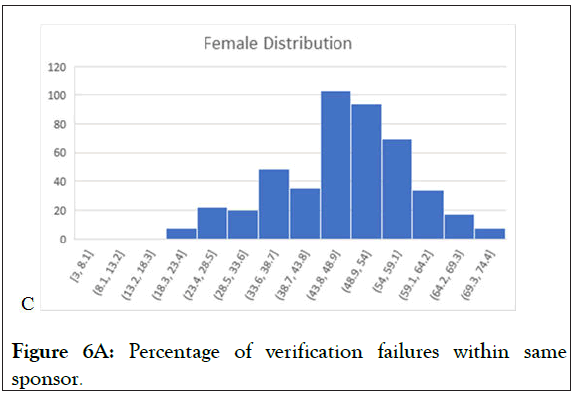
Figure 6A: Percentage of verification failures within same sponsor.
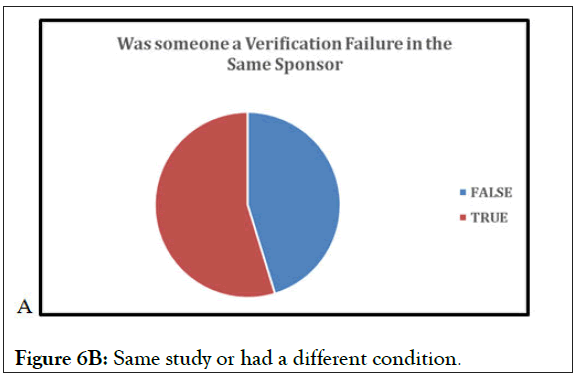
Figure 6B: Same study or had a different condition.
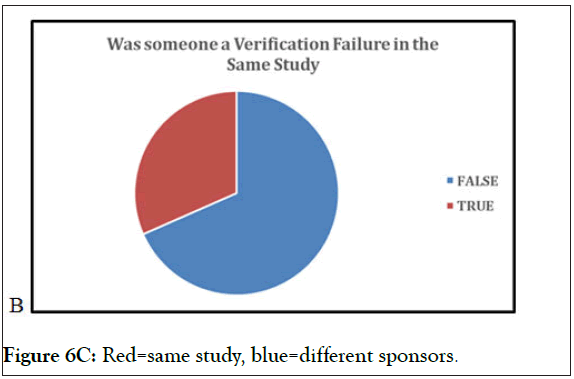
Figure 6C: Red=same study, blue=different sponsors.
In Figures 1 and 2, the sheer volume of potential protocol violations is evident and VCT can help protect trials from this issue. Not all trial subjects are participating for altruistic reasons, and there are more than enough subjects who are “professional”, and travel from study to study for pure financial reasons. Professional subjects will skew the data through fallacious reporting and noncompliance with IP, resulting in potential trial failure and significant financial losses. One of the most significant violations is a washout period violation, wherein a subject does not comply with the requisite amount of time required between trials, most commonly 30 days, before attempting to enroll into another study. A subject must complete the washout period “before randomization to avoid confounding the evaluation of the investigational treatment” [2]. The concern with a washout period violation is that the subject will still have remnants of the prior study’s IP in their system, which could potentially have a dangerous interaction with the new IP or cause skewed data, both of which are undesirable outcomes. However, this is a challenging protocol criterion for a site to verify, as the subject can easily neglect to report prior studies, this is the vacuum that VCT fills. Once a subject is verified, the database will easily pull their prior studies and flag an alert that the subject is in violation, vastly reducing the amount of washout period violations.
VCT can also protect sponsors and their studies from prior IP exposure, another critically important clinical trial criterion. Many protocols will include a list of IPs that they do not wish subjects to have had prior exposure, however this is challenging to verify as prior study history is entirely self-reported by subjects. The inclusion of VCT in the screening process dramatically changes this situation, as with our simple and easy verification process, a subject’s prior IP exposure can be verified against the protocol to ensure that they are eligible. This is crucial because, as stated prior, there are potential interactions between every drug, which could be dangerous if both drugs are still investigational. If the sponsor is unaware of a potential interaction, if the subject has withheld prior IP exposure, the effects could be personally dangerous or could skew results, which is why it is critically important to ensure that VCT is included in studies.
Another alert with a significant number of violations is dual enrollment attempts, when subjects try to enroll in trials concurrently that are potentially not even for the same indications will negatively affect data. The impact of subjects enrolled in multiple trials concurrently can go from missed visits that affect sample collection to different IPs taken at the same time, which is extremely dangerous as outlined earlier. Additionally, the majority of dual enrollment violations tend to come from professional subjects, as they will falsely claim conditions in order to enroll in multiple trials for financial motivation.
The high number of violations seen across the board brings a serious point forward had these subjects been allowed to enroll in their respective studies, those studies could have seen a potentially fatal affect to their data. Specifically, the incredibly high amount of washout period violations could have significantly impacted the study data and potentially resulted in trial failure. Every protocol criterion is present for a reason, and VCT makes it possible to ensure that all are met appropriately.
The financial impact of duplicate subjects and protocol violations
Using the data collected in the program above, we examined the potential financial impact to the sponsors conducting the study. We utilized industry averages for the average cost of each subject to complete a clinical trial. Moore estimated that the price per subject for a clinical trial is around $31,802-$82,362 [3]. In this discussion we estimated that the average price per subject is $40,000 USD, as an averaged published cost per subject per trial. This price was used to estimate the potential estimated money lost by the sponsor if they include ineligible subjects into their trial. By using this price, we approximated the cost savings and Return On Investment (ROI) by utilizing a research subject database registry [4-7].
Therefore, if we evaluate these costs against our chosen studies, the sponsor loses approximately $29,680,000 if ineligible subjects were enrolled. By allowing just subjects violating washout periods, the cost to the sponsor would have been $6,480,000. For these purposes, we have removed all re-screening flags, as those are sometimes allowed to be overridden by the sponsor depending on the protocol, so they could have skewed the data. This is a significant amount of money and could cause massive repercussions if the inclusion of these ineligible subjects had resulted in such poor data that the study needed to be run again.
The point of this study was to demonstrate, using industry averages of Phase II and III CNS studies, what would have happened if the subjects that we protected from entering the study had been enrolled. Academics have found that clinics have been having trouble filling clinical trials with appropriate subjects [5] having the ability to use a database to ensure you are enrolling appropriate subjects only strengthens the value of the data. This example clearly illustrates that, without the database, a sponsor will have massive unnecessary financial costs.
An incredible amount of time, effort, and expense is required to properly design and execute a successful clinical trial. The study protocol describes the critical methodologies and procedures that are required to achieve these goals. One aspect that cannot be controlled or monitored by a protocol is subject behavior as it relates to their study history and past clinical trial participation. Our evaluation of data shows that subjects are not always compliant nor forthcoming. There are attempts to screen more than once, there are age violations, washout period violations, prior exclusionary IP exposure, and other violations that might cause poor quality data in a trial. Sweetman and Zhang reported similar protocol violations and found them to be generally under-reported in clinical trials [8]. Based on the data and analysis earlier in the paper, the high numbers of these violations raise questions as to whether the violating subjects are enrolling in trials “professionally”, which is injurious to the clinical trial data. Enrolling poor quality subjects results in increased placebo rates and issues with data integrity. By utilizing VCT, a Sponsor can ensure that their subjects are verified and are not either enrolled in another clinical trial, still in their washout period or in violation of any other protocol criterion.
VCT provides sites and sponsors the tool to adhere to crucial inclusion and exclusion criteria listed in every protocol. Without the VCT tool, it is nearly impossible for sites to remain compliant, no matter how hard they try. The comprehensive multi-point verification process serves to verify each subject with their own profile within the database, and this will ensure that all the items listed earlier in this paper are appropriately checked. Using this process, VCT enhances and protects all trials, making our database an invaluable tool to improve research subjects’ safety and data quality. This review illustrates the utility of a global research subject database to detect and prevent numerous protocol violations and help prevent a clinical trial from failure.
This paper received no public or private funding.
The Authors manifest no conflict of interests.
Citation: Pinho A, Weingard K, Efros MD (2021) Improving Safety and Preventing Failure in Clinical Trials by Detecting and Preventing Duplicate and Professional Research Subjects: The Case for Use of a Research Subject Database Registry. J Clin Trials. 11:469.
Received: 04-Jun-2021 Accepted: 18-Jun-2021 Published: 25-Jun-2021 , DOI: 10.35248/2167-0870.21.11.469
Copyright: © 2021 Pinho A, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.