
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Editorial Comment - (2014) Volume 4, Issue 5
Background: Lipid-lowering therapy, particularly with hydroxymethylglutaryl-CoA reductase inhibitors (statins), has been shown to significantly reduce morbidity and mortality in patients with and without known coronary artery disease; however adherence is poor particularly among racial/ethnic minorities. Motivational interviewing (MINT) is a patient centered intervention that has been shown to improve self-management through behavior change.
Purpose: The aim is to determine if a call center-based motivational interviewing intervention is more effective than usual care at increasing long term adherence to statins (12 months) among minority subjects.
Methods: Using a randomized design we will compare usual care and MINT. We will include adult black and Hispanic subjects enrolled in a large health benefits company who were newly started in a statin. We will recruit diabetic and non-diabetic subjects to evaluate the impact of MINT in these two distinct populations. We will identify eligible subjects from a large administrative database using a previously validated algorithm. The primary outcome will be medication adherence measured using pharmacy claims as the medication possession ratio. We will define adequate adherence as 80% refill over a period of a year. Our goal is to recruit 800 minority subjects and to have an equal distribution of Black, Hispanics, diabetics and non-diabetics.
Conclusions: The study will evaluate a non-traditional but scalable intervention to curtail the epidemic of lack of adherence to statin therapy.
Keywords: Medication adherence, Minorities, Stents, Motivational interviewing, Cholesterol
Adherence to Hydroxymethylglutaryl-CoA reductase inhibitors (statins) is low in spite of significantly decreasing the risk of cardiovascular events and mortality in patients with hypercholesterolemia [1]. Reasons for the low adherence are not fully understood; however, adverse events, uncertainty about benefits, and mistrust are commonly cited [2-4]. Minorities have a higher prevalence of cardiovascular risk factors [5] and are even less likely to use statins when prescribed [6-8]. This may be related to the presence of additional barriers to adherence such as social, financial, environmental, behavioral and cultural factors; thus, traditional medical models for managing Cardiovascular Disease (CVD) have met with limited success in minority populations. Increasing evidence suggests that nontraditional approaches maybe effective in these groups. Such approaches most typically involve culturally tailored behavioral interventions led by counselors, pharmacists, or lay health educators [9]. This approach may be particularly useful for improving adherence to statins, which seems to require appropriate communication, understanding and follow up [4]. Most patients that discontinue temporarily statins due to adverse events, tolerate well statins long term when rechallenged [10], highlighting the importance of helping patients make informed health decisions when concerns arise.
Among minorities, Motivational Interviewing (MINT) has been shown to improve CVD health behaviors and adherence to CVD medications [11]. However, there are only a limited number of studies rigorously evaluating the effect of such non-traditional interventions on hyperlipidemia among minority groups [5]. The focus of our study to determine the efficacy of a culturally tailored phone based MINT intervention delivered to Latino and African American enrollees living in predominantly minority neighborhoods in Florida, is effective at improving adherence to statins.
Study subjects
Subjects were identified from a Humana claims database of approximately 3.5 million members enrolled in a commercial health maintenance organization (HMO), preferred-provider organization (PPO) or Medicare plan. The database includes a member file containing demographic information such as age, gender, type of insurance, surname, address, Medicare demographic information for each member; a medical file containing up to nine recorded International Classification of Diseases, ninth revision (ICD-9) codes per encounter; a pharmacy file containing all Generic Product Identifier (GPI) numbers of pharmacy-dispensed medications per claim; and a laboratory file which includes results of all laboratory tests performed
Inclusion criteria: To qualify for the study, subjects must have 1) been older than 18 years of age, 2) been identified as Black or Hispanic, and 3) received a new prescription for any of the medications in the statin class. A “new” prescription was defined as the absence of any other statin class pharmacy claim over the 6 months preceding the respective new claim.
Exclusion criteria: We excluded subjects who died or disenrolled from the health plan and self-insured employer groups for whom Humana only provides administrative services. We also excluded subjects who were no longer on statins at the time of the recruitment contact. For the latter group, we collected a short survey to ascertain the reasons for discontinuation.
Identification of racial/ethnic minorities: Our team developed and validated an algorithm to identify individual minority subjects from administrative claims data. This algorithm includes methodologies previously described by others that use census data to identify minority groups [12,13]. Subjects are assigned a likely race/ethnicity according to a 3-step sequential algorithm: 1) matches the last names of all the subjects to the 1990 Census Based Spanish surname list 2) among those who had not already being classified as Hispanic and who were Medicare enrollees, we used the Medicare race code to identify Black race or Hispanic ethnicity, and 3) among those still not classified as either Black or Hispanic we used geocoding techniques, which uses census data on neighborhoods to assign individuals weighted probabilities of being minority based on the racial/ethnic composition of the census tract or block in which the person resides (tracts used for more rural locations). Subjects are classified as Black or Hispanic if they lived in a census block where 75% or more of all persons belonged to that specific minority group. Using self-reported race and ethnicity as gold standard, the algorithm had a positive predictive value (PPV) of 93% (C.I. 85-97) in the identification of individual racial/ethnic minority subjects. By race, the PPV of the algorithm to identify black subjects was 91% (C.I 80-96) and to identify Hispanics 92% (C.I. 75- 99) [14].
Recruitment
We created a recruitment algorithm to automatically query the Humana database on a monthly basis to identify subjects with a new statin prescription during the previous 30 days and who are likely to belong to a minority group based on the previously described algorithm. Given the large number of subjects meeting criteria we then randomly selected 320 subjects per month as a target. The selection process uses a stratified approach to guarantee balanced racial/ethnic and gender distributions. We also stratify for the presence or absence of a diabetes mellitus diagnosis using ICD-9 code 250.xx. The purpose is to have a large enough sample of diabetics to understand if the presence of this diagnosis modifies the adherence behavior or the impact that MINT may have on adherence. Once the identification process is complete, we mailed a brochure to the 320 selected subjects informing them of the study purpose, why they were selected, and provide an opt out prepaid letter to subjects to send if they wish to opt out of the study and not be contacted or if they believe they do not qualify (e.g. not being a minority).
After one week, a bilingual trained counselor contacts potential subjects via telephone. Up to five calls are made at different hours including evenings and weekends to reach an individual before he/ she is considered as “unable to locate”. Once subjects are reached, the counselor explains the study, screens patients for eligibility using computer assisted interviewing, explains to subjects they have an equal chance of being in either arm of the study, and explains that participation is voluntary. We inform the potential participants that only their insurance carrier has access to their medical information with identifiers (as they have already), and that Humana will only send de-identified data to researchers. We also inform subjects that for their time and participation in the study, they will receive a $10 gift card for completion of the initial assessment at baseline and another upon completion of the follow up call at the end of the study ($20 total). If patients are deemed eligible and interested in the study, then we obtain verbal informed consent and enroll the subject in the study. We ask non-participants to give their reasons for not participating and to respond a very short survey so that we can compare the participating and non-participating groups. Figure 1 shows the recruitment strategy.
Interventions
Once screened and evaluated at baseline by telephone contact, the recruitment team provides the unique ID numbers of those who agreed to participate to the statistician who used a block randomization scheme with varying sizes of blocks in a 1:1 and stratified for race/ethnicity to assure balance of one of two treatment arms: MINT or Usual Care.
Motivational interviewing: MINT was chosen as the counseling strategy for maintenance of behavior change and improvement of statin adherence due to its focus on self-determination and self-management as key mediators of successful behavior change. MINT is a well-known, scientifically tested behavioral counseling strategy developed as an amalgamation of principles drawn from several theoretical paradigms, the most important of which are Self-Determination Theory, Patient- Centeredness, Self-Efficacy Theory, and the Stages of Change Model [15]. It is defined as a directive, patient-centered approach to counseling designed to motivate people for change by helping them to recognize and resolve the discrepancy between their present behavior and their future personal goals and values. It helps patients clarify goals, explore their perceived barriers to recommended behavior change, and make commitments to such change. To illustrate, if a patient is considering stopping a statin due to the perception of an adverse event, MINT can be used to explore the origin of the concerns, consider the information available about the risk of the adverse event versus the risk of hypercholesterolemia, re-establish long term health goals and elicit an action plan to resolve the ambivalence about the treatment. In summary, MINT attempts to improve self-management skills so the individual can align health goals and behaviors and resolve barriers or concerns.
MINT is guided by five basic principles: 1) expressing empathy; 2) developing discrepancy between patients’ goals and their current health behavior; 3) avoiding argumentation by assuming that the patient is responsible for the decision to change; 4) “rolling with resistance” rather than confronting or opposing it; and 5) supporting self-efficacy and optimism for change. In clinical practice, the concepts and theories in MINT are achieved with the use of two basic techniques: 1) Reflective listening, (in which the provider allows patients to voice their perspectives of the problem), and 2) Eliciting self-motivational statements.
Results from a randomized clinical trial by Ogedegbe et al. [11] suggest that MINT may be effective in improving adherence to medications. Ogedegbe et al. [11] tested the effect of a practice-based MINT counseling vs. usual care on medication adherence in 190 hypertensive African Americans recruited from two community-based primary care practices in New York City. The study showed that while baseline adherence was similar, the post-treatment adherence rates were 43% and 57% in the usual care and MINT groups, respectively (P=0.03) [11]. Based on these findings we proposed to compare the effectiveness of MINT as a tool to inform and activate change in behavior around adherence to statins in minority populations. We will recruit diabetic and non-diabetic subjects to evaluate the impact of MINT among populations with potentially different perceptions of risk and self-management skills. The goals of our intervention were to amplify the awareness of autonomy, create an empathic relationship, help subjects resolve ambivalence, and coach them towards expressions of commitment that can be reinforced and supported via self-efficacy.
The MINT intervention in the current study consisted of 6-to- 9 telephone encounters between a bilingual counselor trained in Motivational interviewing and a minority subject who recently received a prescription for statin. All subjects in the MINT arm are to be contacted every 3 months to complete 6 encounters; however subjects who were not filling medication appropriately may receive additional calls if agreed with the counselor during a scheduled call.
Each telephone encounter lasts about 15-20 minutes and has a patient centered approach with the following basic structure and goals: 1) Establish a connection and reinforce autonomy via open ended questions regarding the health status or wellbeing of the subject. The counselors use open ended questions regarding the issues regarding their statins use, followed by statements that reinforce the autonomy of the subject, to establish an empathetic connection with the subject via reflective listening; 2) Collection of a self-reported adherence measure, the 8-item Morisky Medication Adherence Scale (MMAS-8) to have an objective measure of adherence to use as springboard for discussion. 3) Elicit expressions of ambivalence subjects may have regarding taking their statin (e.g. fear of a bad outcome versus fear of side effect). The counselor empathized with the feeling and alternated presenting ideas for resolving the ambivalence with asking what the subject thinks will make him/her resolve the ambivalence in the short term; 3) Coach the subject towards expressions of commitment since it is predictive of change.
All study calls are audio-recorded. To assess fidelity of the intervention, a trained MINT specialist (LHF) listened to a random sample of 10% of the MINT calls. Calls are assessed for the ‘Spirit’ of MI, the degree of empathy and direction, and the actual MI techniques employed by the counselors. Examples of these techniques include open-ended questions, reflective listening, and asking permission prior to providing advice. The MI specialist meets once per month with the counselors, to provide feedback on the calls, make recommendations about competencies that needed development, and identify where progress was evident.
Usual care: Subjects randomized to usual care receive a brochure every 6 months for a total of 3 mailings. The brochure highlights the importance and impact of controlling cardiovascular risk factors, tips to improve statin adherence, smoking cessation strategies, and public services. The brochures we sent are: 1) You can manage your cholesterol: A guide to low cholesterol living (Krames Patient Education), 2) How can I quit smoking (AHA), 3) How can I lower high cholesterol (AHA), 4) Signs of Heart Attack (AHA). The subjects randomized to this arm are contacted by phone only at the beginning and end of the study. We will collect their claims data after they complete the 12 months of follow-up. For retention purposes, along with the brochures we mailed them reminders of study participation and requests to update contact information every six months.
Outcomes
Primary outcome: The primary outcome is Medication Possession Ratio (MPR) for statin medication equal or above 0.80. A validated formula [16], described below, will be used to calculate the continuous MPR to then be dichotomized. This allows for the creation of a more clinically meaningful binary variable: MPR equal to or above 0.80 (considered adequate adherence or drug availability during 80% or more of the therapy time) and MPR below 0.80 (non-adherence or drug availability during less than 80% of the therapy time). An additional advantage is that the MPR correlates well with the LDL value and that the MPR is higher among those with the LDL at target levels [17].
Definition of medication possession ratio (MPR): The Medication Possession Ratio (MPR) is a continuous multiple interval measure of medication availability validated to estimate medication adherence [16]. The MPR is defined as the sum of the days’ supply of medication divided by the number of days between the first fill and the last refill plus the days’ supply of the last refill.
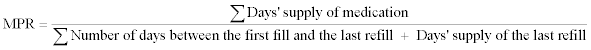
This calculation usually results in a ratio less than 1.0 if there are lapses in prescription refilling. This method could result in overestimation of the MPR in early refillers leading to a MPR greater than 1.0. The MPR in such a case will be truncated at the maximum value of 1.0, indicating the potential for perfect adherence. Using this methodology, an accepted definition of adequate adherence is an MPR of 0.80 or above which means that subjects had the medication available 80% or more of the therapy time.
Secondary outcomes: Self-reported statin medication adherence was compared between the two intervention arms using the 8-item Morisky Medication Adherence Scale (MMAS-8). The MMAS-8 is a generic self-reported, medication-taking behavior scale in which a specific health issue is inserted as the “health concern” in each of the questions. The MMAS consists of eight items with a scoring scheme of “Yes”=0 and “No”=1. The items are summed to give a range of scores from 0 to 8. In addition, MPR was reviewed as a continuous variable to examine more subtle variations in adherence among subgroups [18,19].
Claims data was explanatorily examined for myocardial infarction and stroke based on ICD-9 codes 410.xx 434.xx and 435.xx as well as mortality. Mortality data was obtained by matching the social security numbers of all subjects against the social security database which has significantly shorter lag time than the National Death Index. Discontinuation of statins was based on the date of last filled statin prescription if it occurred more than the number of days supplied plus half of the number of days supplied before the end of follow up.
Given the nature and objectives of this James and Esther King Biomedical Research Program, we considered it important to collect and analyze smoking history information as well as to explore the impact of MINT on smoking behaviors among minorities. The results can inform larger trials that have a primary outcome of smoking cessation among racial/ethnic minority groups. Among smokers we examined self-report of smoking cessation attempts, approximate date of last cigarette, average number of smoking days over past 30 days, average number of current daily smoked cigarettes, and frequency of smoking cessation aids used.
Covariates: Although our study design was expected to balance unmeasured confounders, there are some that may play a large role in adherence that we collected to possibly adjust for in our analysis. The included demographics (age, gender, urban/rural [census tract], income [zip code median], marital status, education); depression (captured by ICD-9 claims code or positive screen for depression at baseline using the PHQ-9); total pill burden (total number of pills has been related to adherence to medications); type of insurance (Medicare advantage, plans with high deductible or copays [>$500 deductible or >20% visits co-payment, or >20% cost co-share for certain medications]); acute myocardial infarction (captured by ICD-9 410.xx) or acute stroke (434. xx and 435.xx ); diabetes mellitus (defined as ICD-9 250.xx); Charlson co-morbidity score (using our claims based co-morbidity algorithm); and hypertension ( ICD-9 codes 401xx-405xx).
Statistical analysis
Sample size: The MPR is the standardized method used to measure adherence in claims data, and reaching and maintaining an MPR of 0.80 or more was considered appropriate. Preliminary analyses of MPR for statins using a database of Humana members who received a coronary stent show that the proportion of subjects with MPR >= 0.80 is 58% at one year. We hypothesized that in the MINT group at least 73% of subjects will reach an MPR of 0.80 while in the usual care group that proportion will remain at or below 58% Our sample size estimates showed that, using a conservative 2-sided test at the 5% alpha level and a 90% power, we will be able to detect as significant a 15% difference (73%-58%) at 12 and at 24 months with a sample of 209 in each study group. Under the same conditions, but with an 80% power, we need 157 participants in each group. We would aim to recruit 400 African American subjects and 400 Hispanics per group to have the necessary power even after attrition to do within race/ethnicity comparisons of the MINT intervention against usual care and evaluate the net effect in each ethnic group. Similarly each arm will have balanced number of diabetics and non-diabetics to be able to explore an interaction between presence of diabetes and MINT.
Chi-square analyses will be used to compare the proportion of subjects that reached an adequate MPR of 0.80 or higher in both intervention groups. We expected that, as a result of the randomization process, the two intervention groups were comparable at baseline. However, if this was not the case or if we detected attrition biases, a logistic regression analysis approach was used with adequate adherence to statins (i.e. MPR >= 0.80 vs <0.80) as the dependent variable, and intervention group as the main independent variable. Analyses will be performed using race/ethnicity as covariate and also stratified by race/ ethnicity.
Statistical techniques similar to those described for the primary outcome will be used for analyses of the secondary outcomes. For example, we compared the two intervention groups with respect to the proportion of subjects who discontinue the statin, mean Morisky score at 12 months and 24 months, change in Morisky scores from baseline to end of the trial and among the smokers we will compare the proportion of subjects who quit smoking for any length of time and for more than 6 months, number of quitting attempts, average number of smoking days over past 30 days , average number of daily cigarettes, frequency of use of smoking cessation aids.
Additionally we used claims data to collect, report, and compare incidence of myocardial infarction, stroke and all-cause mortality separately and as composite outcomes among both groups at 24 months of follow up.
Statins are potent cholesterol lowering medications shown to prevent primary and secondary CVD morbidity and mortality [20-22]. These benefits outweigh the risks as serious adverse events are rare and frequently associated to the concomitant use of medications affecting statin metabolism [23]. To date, much of the existing work in the area of cholesterol lowering medication is aimed at identifying newer more powerful agents or lowering the targets for intervention. Yet, as we and others have shown, one major existing gap in knowledge are interventions that can address the largest barrier to cholesterol lowering initiatives which are the low medication adherence rates even after the occurrence of acute cardiovascular events [24]. Poor adherence to statins among high risk groups has been associated high cardiovascular and all-cause mortality [25]. A simulation study showed that improving the adherence to statins would have a much more profound impact at reducing CVD events and costs than lowering the low density lipoprotein (LDL) threshold [26]. Thus, this translational and health disparity research study will provide timely information on a low cost approach to translate important clinical discoveries into every day clinical care. This is of particular importance among minority populations who have a higher prevalence of CVD risk factors, are less likely to receive evidence based interventions and more likely to experience worst CVD outcomes [5,24,27-29].
Motivational interviewing has been shown to improve medication adherence among vulnerable groups such as those receiving treatment for HIV infection [30,31] or minorities with Hypertension [6]. Based on these data we expect that phone based MINT helps patients to identify their values and goals, to realize the benefits of statin therapy and to formulate concrete questions and a strategy to resolve them when experiencing ambivalence.
Our innovative approach capitalizes on information already collected for administrative purposes providing a cost-effective method of identifying research subjects, recruitment strategy and outcome assessment. This academic-insurance company partnership is also beneficial because it helps inform and direct quality of care improvement programs which are now becoming commonly employed among private insurers. This approach allows insurers, most of whom have existing behavioral interventions units, to target high risk groups for a relatively low cost. While our study is on racial and ethnic minorities, if proven effective, this strategy could also be used for other vulnerable high risk groups such as subjects with low socioeconomic status, low education, and the elderly. Future studies that could stem from our data will be able to measure the cost effectiveness of our behavioral intervention on the prevention of cardiovascular outcomes in groups at risk such as racial/ethnic minorities. At a time when our country is exploring ways to expand coverage or benefits to underserved populations, this study can also inform policy makers on the impact of a scalable telephone based intervention on medication adherence in an important chronic cardiovascular condition.
Our study has significant strengths due to its innovative study design. However, there are also some limitations. First, the attrition rates usually present in private health plan, the low risk CVD population used and the short follow-up prevent us from powering the study to compare the impact of the interventions on clinical outcomes, but observational studies have already established that lack of appropriate adherence to statins increases significantly the risk of MI, stroke and death among those with cardiovascular risk factors or diabetes. Second, we did not include other vulnerable groups such as NHWs with low income or a rural place of residence due to the sample size implications of having such a diverse cohort. Third, the use of statin as a class will limit our ability to explore for different effect size on clinical outcomes for each individual medication, however the effect size of most statins have been extensively described in clinical trials.
The study was sponsored by the James and Esther King Biomedical Research Program Grant numbers 1KG-11 and 4KB13.