
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2023)Volume 13, Issue 3
Background: Urticaria negatively impacted patients’ quality of life, day to day activities and productivity at work place. The present study evaluated effectiveness of Levocetirizine on symptom control, quality of life and work productivity in patients with urticaria.
Methodology: The study was a prospective, open-labelled, single-arm, observational study among patients with chronic urticaria. It was initiated after necessary approval from the Institutional Ethics committee. Levocetirizine was prescribed as 5 mg or 10 mg once a day evening oral dose for consecutive 7 days at bed time. Urticaria symptom control such as size, frequency of wheal, duration of pruritis, quality of Life using SF-12 scale, degree of sleepiness applying Stanford Sleepiness Scale and work output and activity impairment using WPAI questionnaires were assessed.
Results: Following administration of oral Levocetirizine, effective symptom control, as indicated by significant reductions in the mean symptom scores like size, frequency of wheal and duration of pruritis were observed. While quality of life significantly improved, there was no change in degree of sleepiness at morning and afternoon hours. However, Levocetirizine produced modest degree of somnolence at night-time though it did not affect quality of life as compared to baseline.
Conclusion: Levocetirizine alleviated clinical symptoms while having no significant adverse effects on daytime somnolence and quality of life among patients with urticaria.
Levocetirizine, Urticaria, Anti-histaminics, Quality of life, Work productivity, Work Productivity and Activity Impairment (WPAI), Sleepiness scale
Chronic urticaria is a common skin condition which is one of the major diagnostic and therapeutic challenges in allergology. It is a disabling disease because of persistent clinical symptoms, the unpredictable course and its negative influence on quality of life [1]. Because of emotional distress, patients with chronic urticaria often suffer from anxiety, depression, and somatoform disorders. As a result, the societal burden of the condition is immense in terms of both direct and indirect health care costs [2].
Several mediators like histamine, inflammatory cytokines, chemokines, leukotrienes, prostaglandins and platelet-activating factor are responsible for vasodilation, sensory activation, plasma leakage and recruitment of cells at the site of lesions in urticaria. In most cases, histamine plays a leading role in the pathogenesis. That is why antihistamines remain a cornerstone of effective therapy [3].
Antihistamines alleviate the symptoms of urticaria, including size and frequency of wheal as well as duration of pruritis [4]. Current international guidelines propose that second generation nonsedating antihistamines cetirizine, desloratadine, ebastine, fexofenadine, levocetirizine, loratadine and rupatadine constitute the first line of treatment for all forms of urticarial [5].
Levocetirizine is a single-isomer second generation antihistamine with proven efficacy in chronic urticaria as documented in clinical studies [6]. Levocetirizine demonstrated higher efficacy and longer duration of action in inhibiting histamine-induced wheal formation as compared to ebastine (10 mg), fexofenadine (180 mg), mizolastine (10 mg), and loratadine (10 mg) [7]. Compared with cetirizine, lesser degree of somnolence was observed with levocetirizine which may be attributed to minimal crossing of blood-brain barrier and small volume of distribution. Lack of cardiotoxicity and mild adverse effects makes the drug acceptable [8].
Treatment of urticaria should be tailored to individual patient characteristics and choice of antihistaminic should be guided by evidence of efficacy, tolerability and safety. The other criteria’s for rational selection includes indicators, better quality of life, work productivity and compliance by patients.
The efficacy of levocetirizine to treat chronic urticaria is well established among adults and children above 6 years of age. According to a systematic review, levocetirizine produced lesser degree of sedative effects as compared to first-generation antihistamines [9]. However, comprehensive studies evaluating the effects of levocetirizine interventions on symptoms of urticaria, quality of life, adverse effects like 24-hour somnolence and work productivity are sparse.
Hence, the present study was conducted to assess if oral levocetirizine could help in better urticaria symptom control without compromising work performance, overall quality of life and drug adherence.
Study design
The Department of Otolaryngology and Dermatology, Dr. D Y Patil Medical College and Hospital, Navi Mumbai, Maharashtra, India, conducted the prospective, open-label, single-arm, observational study on patients of urticaria.
This study was carried out in accordance with ICH-GCP E6 including informed consent of the subjects. The Institutional Ethics Committees (IEC) examined and approved all study documents prior to the start of the study.
Study participants
For the study, 55 patients with urticaria were included. An effect size of 0.5, a power of 99%, a two-sided significance level of 95%, and a non-centrality parameter value of 4.281 were used to compute the sample size.
Test product, dose, mode and timing of administration
Patients with urticaria received oral Levocetirizine, 5 mg or 10 mg OD evening dose, consequently for 7 days prior to going to bed.
Treatment compliance
Patients with urticaria self-administered the Levocetirizine at bed time. Patients were to be excluded from analysis of results if they needed to be weaned off the treatment or given alternative prescription for delayed recovery or for any other reason. Either in-person or over the phone follow-up was conducted.
Inclusion and exclusion criteria
The study included male or female patients above 18 years of age with Chronic Idiopathic Urticaria (CIU) episodes with hives of characteristic wheal and flare, occurring at least three times a week for a period of at least 6 weeks during the previous 3 months without an identifiable cause. All these patients experienced at least 3 days of moderate or severe pruritus and wheals at the time of the recruitment.
Subjects with any abnormal clinical or laboratory findings, history of alcohol or drug use, known hypersensitivity to any of the components in the formula used in the study were excluded. The other criteria for exclusion included pregnancy or risk of pregnancy, lactating mothers and other forms of urticaria, such as physical, drug-induced, acute, or cholinergic urticaria. Patients with any systemic disease or dermatologic conditions that interfered with evaluation of symptoms or on concomitant topical corticosteroids were also excluded.
Efficacy end point
The following scales were used to assess efficacy variables during the study:
• Frequency of wheals
• Size of the wheals
• Duration of Pruritus
• Self-reported WPAI assessment during working days: The Work Productivity and Activity Impairment (WPAI) questionnaire calculates absence from work and the activity impairment due to a specific health issue over the previous seven days [10].
• Self-reported Stanford sleeplessness scale: The Stanford sleepiness scale rating was used to gauge how sleepy the study participants were on average [11].
• Quality of life: The SF-12 is a self-reported outcome measure that evaluates how one's health impacts day-to-day activities. Higher scores, in the scale from 0 to 100, indicated better quality of life [12,13].
Study evaluations and follow up time points
Patients were screened for eligibility on Day 0 prior to enrolment in the study. The frequency of wheals, size of wheals and duration of pruritus was assessed at baseline before starting treatment and at scheduled intervals of Day 1 (24-hour), Day 3 (73 hours), and Day 7. Quality of life, Work productivity, activity impairment as well as degree of sleepiness in the morning, afternoon, and night were measured before and after treatment with Levocetirizine.
Data of patients who completed the study for a period of 7 days/ week were considered for the final analysis.
Safety endpoints
All Adverse Events (AEs), whether previously known or otherwise, were documented at each assessment time points along with information about their nature, severity, course of action, duration and severity were recorded.
Statistical analyses
Results were expressed as mean ± SD for nominal data and as proportions for categorical data. P<0.05 was considered as statistically significant. As the data was on an ordinal scale of measurement, statistical analysis was performed using Wilcoxon Signed Rank Test for calculation of significance for assessment parameters post Levocitrizine treatment compared to baseline values.
Demographic and other baseline characteristics
The frequency distribution based on demographic characteristics of patients with urticaria is summarized in Table 1. The cohort of patients with urticaria consisted of more female patients (32, 58.18%)) as compared to male patients (23, 41.81%)). A greater frequency of patients was between 31-40 years of age (20, 36.36%). Their height was between 140 cm-160 cm (29, 52.73%), had their weight between 50 kg-60 kg (29, 52.73%), and had a BMI between 18.5 kg/m2-24.9 kg/m2 (50, 90.91%).
| S. No | Parameters | No of Patients | Percentage |
|---|---|---|---|
| 1 | Age Group (years) | ||
| 18-20 | 0 | 0 | |
| 21-30 | 15 | 0.2727 | |
| 31-40 | 20 | 0.3636 | |
| 41-50 | 7 | 0.1273 | |
| 51-60 | 9 | 0.1636 | |
| 61-70 | 2 | 0.0364 | |
| >70 | 2 | 0.0364 | |
| 2 | Height (cm) | ||
| 140-160 | 29 | 0.5273 | |
| 160-180 | 25 | 0.4545 | |
| >180 | 1 | 0.0182 | |
| 3 | Weight (kg) | ||
| 40-50 | 11 | 0.2 | |
| 50-60 | 29 | 0.5273 | |
| 60-70 | 12 | 0.2182 | |
| 70-80 | 2 | 0.0364 | |
| >80 | 1 | 0.0182 | |
| 4 | Body Mass Index (kg/m2) | ||
| <18.5 | 1 | 0.0182 | |
| 18.5-24.9 | 50 | 0.9091 | |
| >25 | 4 | 0.0727 |
Table 1: Frequency Distribution according to Patients Demographic Characteristics.
The study participants had normal systemic examination findings. None of the urticaria patients reported hypertension, diabetes, Chronic Obstructive Pulmonary Disorder (COPD) or asthma as comorbidities.
Efficacy among patients with urticaria
Frequency and size of Wheals: A significant (p-value=0.000000476618) decrease in the mean score for frequency of wheals was observed following treatment with Levocetirizine as compared to baseline values. The percent change in mean scores from baseline on Day 1 (24- hour), Day 3, and Day 7 were found to be 4.64%, 15.23% and 25.17% respectively (Figure 1, Table 2).
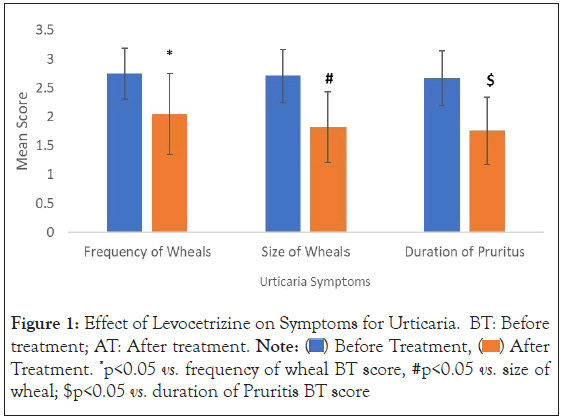
Figure 1: Effect of Levocetrizine on Symptoms for Urticaria. BT: Before
treatment; AT: After treatment. Note:  After Treatment. *p<0.05 vs. frequency of wheal BT score, #p<0.05 vs. size of
wheal; $p<0.05 vs. duration of Pruritis BT score
After Treatment. *p<0.05 vs. frequency of wheal BT score, #p<0.05 vs. size of
wheal; $p<0.05 vs. duration of Pruritis BT score
| S. No | Symptoms | Mean (± SD) | Percentage change |
|---|---|---|---|
| 1 | Frequency of Wheals | ||
| Before Treatment | 2.75 (± 0.44) | - | |
| Day 1 | 2.62 (± 0.53) | 4.64 | |
| Day 3 | 2.33 (± 0.61) | 15.23 | |
| Day 7 | 2.05 (± 0.70) | 25.17 | |
| 2 | Size of Wheals | ||
| Before Treatment | 2.71 (± 0.46) | - | |
| Day 1 | 2.53 (± 0.50) | 6.71 | |
| Day 3 | 2.07 (± 0.57) | 23.49 | |
| Day 7 | 1.82 (± 0.61) | 32.86 | |
| 3 | Duration of Pruritus | ||
| Before Treatment | 2.67 (± 0.47) | - | |
| Day 1 | 2.56 (± 0.50) | 4.08 | |
| Day 3 | 2.11 (± 0.57) | 21.09 | |
| Day 7 | 1.76 (± 0.58) | 34.01 |
Table 2: Time course of changes in mean scores for wheals parameters and duration of Pruritis following Levocetirizine administration.
As compared to baseline values, there was a significant (p-value=0.000000004709) decrease in the mean score for size of wheals was observed with Levocetirizine administration. The change in mean scores observed on Day 1 (24-hour), Day 3, and Day 7 was 6.71%, 23.49%, and 32.86% respectively as compared to baseline scores (Figure 1, Table 2).
Duration of Pruritus: Duration of pruritus significantly decreased (p-value=0.000000002906) with Levocetirizine treatment. The Day 1 (24-hour), Day 3, and Day 7 mean scores (percent change from baseline) were 2.56 (4.08%), 2.11 (21.09%), and 1.76 (34.01%) respectively as compared to 2.67 observed before treatment (Figure 1, Table 2).
Quality of life: On a 12-item short form health survey (SF-12), the mean score post treatment was found to be 25.55 (± 1.82) compared to baseline of 26.15 (± 1.74). A significant decrease in (p-value=0.037467256059) in post-treatment value (percent change of 2.29%) was observed in the mean SF-12 score as compared to the pre-treatment score (Figure 2).
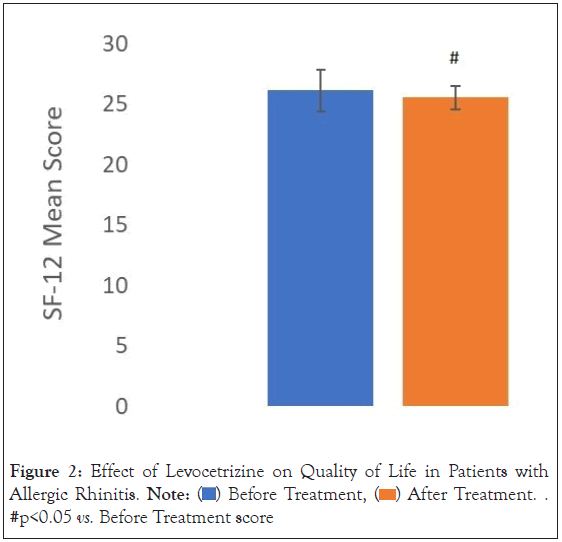
Figure 2: Effect of Levocetrizine on Quality of Life in Patients with
urticaria. Note:  After Treatment. #p<0.05 vs. Before Treatment score
After Treatment. #p<0.05 vs. Before Treatment score
The Stanford sleepiness scale: Change in mean scores for sleepiness at morning, afternoon and night among urticaria patients on therapy were found to be 8.55%, 1.92% and 12.68% respectively. No significant change in the corresponding p-values for degree of sleepiness at different time of the day were observed (p-value=0.462262134534 (morning) vs. 0.226213453400 (afternoon)). However, the corresponding p-value for degree of sleepiness at night was observed to be less than 0.05 (p-value=0.000000002215) was significant during night on levocetirizine therapy (Figure 3).
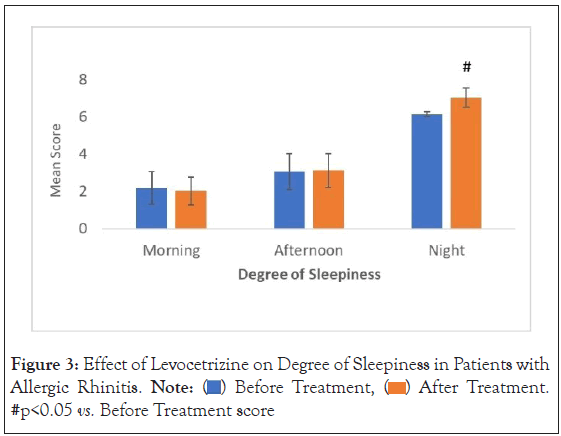
Figure 3: Effect of Levocetrizine on Degree of Sleepiness in Patients with
urticaria. Note:  After Treatment. #p<0.05 vs. Before Treatment score
After Treatment. #p<0.05 vs. Before Treatment score
Work Productivity and Activity Impairment Questionnaire (WPAI): The mean WPAI score following treatment with Levocetirizine showed a percent change of 0.54% (reduced from 52.64 to 52.36) after treatment. The corresponding p-value for WPAI score was found to be statistically insignificant (p-value=0.602) (Figure 4).
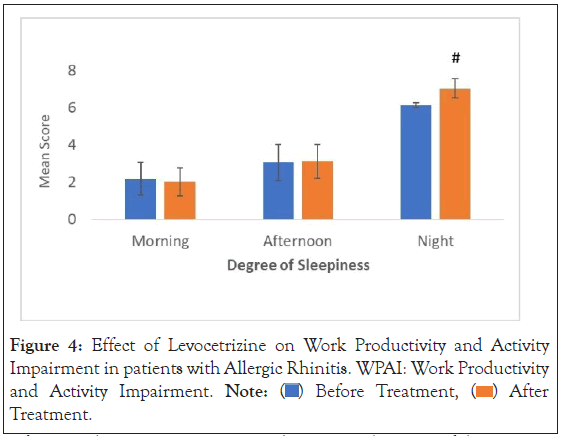
Figure 4: Effect of Levocetrizine on Work Productivity and Activity
Impairment in patients with urticaria. WPAI: Work Productivity
and Activity Impairment. Note:  After Treatment.
After Treatment.
Safety results among patients with urticarial: None of the patients enrolled within urticaria cohort reported any post treatment adverse drug reactions/adverse events. There was no death, serious adverse events, or other adverse events reported during the course of the study. No significant change in vital sign parameters (systolic blood pressure (p=0.383), diastolic blood pressure (p=0.920), pulse rate (p=0.094), respiratory rate (p=0.588), and body temperature (p=0.442)) was observed (Table 3).
| Parameter | Mean | Standard Deviation | Percent Change | |
|---|---|---|---|---|
| 1 | Diastolic Blood Pressure (mm Hg) | |||
| Before treatment | 117.42 | 5.67 | - | |
| Day 1 | 118.4 | 4.59 | 0.84 | |
| Day 3 | 119.75 | 3.31 | 1.98 | |
| Day 7 | 118.38 | 4.93 | 0.82 | |
| 2 | Pulse Rate (beats per minute) | |||
| Before treatment | 78.44 | 4.98 | - | |
| Day 1 | 77.96 | 5.07 | 0.6 | |
| Day 3 | 78.47 | 4.87 | 0.05 | |
| Day 7 | 77.2 | 5.24 | 1.58 | |
| 3 | Respiratory Rate (breaths per minute) | |||
| Before treatment | 78.47 | 7.99 | - | |
| Day 1 | 75.62 | 5.96 | 3.64 | |
| Day 3 | 76.22 | 6.05 | 2.87 | |
| Day 7 | 75.76 | 5.6 | 3.45 | |
| 4 | Body Temperature (°F) | |||
| Before treatment | 15.6 | 1.5 | - | |
| Day 1 | 15.91 | 1.66 | 1.98 | |
| Day 3 | 16.42 | 1.98 | 5.24 | |
| Day 7 | 16.69 | 2.36 | 6.99 |
Table 3: Vital Signs, Physical findings among urticaria patients following Levocetirizine administration.
Chronic urticaria negatively impacts patient’s quality of life, vitality, sleep, mobility and social life. These patients often suffer from anxiety, depression which contribute to decreased work performance, societal burden and health care costs. Patients with urticaria present with redness, swelling, and itchy plaques on the skin and mucous membrane. Pruritus happens to be most bothersome, particularly at night when it causes sleep disturbances [14,15]. Such sleep disturbances lead to chronic fatigue and drowsiness on the following day, with a direct impact on Quality of Life (QoL), physical and emotional well-being. In addition, it has been reported that pruritic skin diseases impair workplace productivity and daily activity. Effective and quick symptom control can reduce the number of days lost at work resulting in improvement of overall quality of life as well as productivity [3].
There is good evidence that antihistamines are helpful in the short and intermediate-term suppression of urticaria. They decrease in itching, reduce the number, size, duration of wheals and flares (erythema), thereby improving quality of life. The second-generation antihistamines are more potent and produce long lasting effect with least side effects. These have high affinity for H1 receptors and cause minimum sedation, common with older generation antihistamines as the brain-blood barrier is less permeable to the former [16]. Therefore, second generation antihistamines are preferred over the first-generation ones [17,18]. However, there are paucity of studies which comprehensively evaluate the effect of Levocetirizine administration on symptom control, somnolence, quality of life, work performance and daily activities.
Efficacy conclusions
In patients with urticaria, significant improvement in frequency of wheals, size of wheals, and duration of pruritus post treatment with Levocetirizine was established. There were similar studies which demonstrated effectiveness of levocetirizine in attenuating symptoms of urticaria. In several other studies levocetirizine has been shown as an effective and satisfactory therapy for patients of urticarial [2,19,20]. The superiority of levocetirizine over loratadine for chronic urticaria was reported in a study [21]. It was also demonstrated that levocetirizine is comparable to the newer antihistaminic drug Bepotastine in respect of its effectiveness with an edge in terms of side-effect (sedation during day time) [19]. Another study documented that Bastine 20 mg was found to have superior efficacy for treatment of Urticaria as compared to Ebastine 10 mg but with levocetirizine 5 mg the results were almost similar [22]. There are several studied that have used high-dose levocetirizine or use of combination therapy with Mon-teleukast for the treatment of refractory chronic urticarial [23-25]. However, in the present study all the patients responded better clinically and showed improvement in the symptoms of urticaria including duration of pruritis.
Besides pruritus and papules, other factors are equally relevant for patients with chronic urticaria, such as sleep disorders quality of life, drug-related adverse effects, efficacy and work productivity. Thus, evaluating the urticaria symptoms only may not be sufficient. A holistic evaluation of the patient is desirable for a better understanding impact of disease and therapeutic effect of drugs.
Chronic urticaria is a debilitating disease that considerably affects health-related quality of life. Pruritus also causes consider discomfort. Multiple lesions, depending on their number and location are responsible for the poor quality of life for patients of chronic urticaria. Consistent with published literature low mean SF-12 scores suggesting poor quality of life in patients with urticaria before initiating levocetirizine treatment has been established. In the present study, following treatment with Levocetirizine, a significant improvement in quality of life (decrease in mean SF-12 score) was observed as compared to the pre-treatment score. Several studies concurred with the findings of our study [26,27].
Sleep disorders such as insomnia, fatigue and drowsiness due to pruritus or side effects from antihistamines are frequently observed. In the present study, patients on levocetirizine did not report sleepiness in the morning and afternoon though increase in somnolence was noted at night time. Tzanetos et al., 2011 reported that most patients with a perceived history of sedation with cetirizine, were tolerating levocetirizine better [28]. However daytime sedation with levocetirizine was observed by Sil et al. [20]. Day time sedation is undesirable as it interferes with subjective well-being and daily life; compromising the work performance. No such adverse effect was observed in the present study.
No significant change was seen post-Levocetirizine administration in the mean WPAI score, an indicator of Work Productivity and Activity Impairment. It can be concluded that the administration of Levocetirizine did not significantly affect work efficiency and daily activity among patients with urticaria. Evidence is less clear on the impact of Levocetirizine on work productivity and daily activities of urticaria patients which other studies did not address. Therefore, the results in the present study add to the existing knowledge on safety and efficacy of treating urticaria with Levocetirizine which was well tolerated as well as cost effective compared to newer class of antihistamines.
Patients of urticaria did not experience any significant change in systolic blood pressure, diastolic blood pressure, pulse rate, respiratory rate, and body temperature following treatment with Levocetirizine. No treatment-emergent adverse events were reported either. Hence it is concluded that Levocetirizine provided symptom control, improves quality of life minimising somnolence during morning and afternoon time among patients of urticaria.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Godse K, Singh M, Rathod R, Paspulate A, Veligandla KC, Rathod R, et al. (2023) Impact of Levocetirizine on Quality of Life and Daily Activities in Patients with Chronic Urticaria. J Clin Trials. 13:527.
Received: 11-Apr-2023, Manuscript No. JCTR-23-23433; Editor assigned: 13-Apr-2023, Pre QC No. JCTR-23-23433(PQ); Reviewed: 27-Apr-2023, QC No. JCTR-23-23433; Revised: 04-May-2023, Manuscript No. JCTR-23-23433(R); Published: 11-May-2023 , DOI: 10.35248/2167-0870.23.13.527
Copyright: © 2023 Godse K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.