
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2025)Volume 16, Issue 2
Introduction: B1a lymphocytes constitute components of the innate immune response. They maintain their existence by self-renewal of preexisting cells, a process that declines with age.
Objective: Determine the effect induced by Biomodulin T® (BT) on B1a lymphocytes in older Cuban adults.
Methods: 30 older adults were studied. The included subjects received 1 bulb of BT intramuscularly, twice a week, for six weeks. The subpopulations of B1a lymphocytes CD19+CD5+, CD20+CD5+ and CD19+CD20 were determined. Descriptive statistics were evaluated: Median, Standard Deviation (SD) and Odds Ratios (OR) after six weeks and six months after completing the course.
Results: The median percentages and absolute counts of B1a CD5+CD20+ lymphocytes increased after 6 weeks of treatment with BT (0.955 vs. 2.110, SD 6.017 vs. 2.380) and (17.02 vs. 40.58, SD 80.09 vs. 39.70), with significance statistics, p=0.026 and p=0.014, respectively. The percentage and absolute count of lymphocytes that coexpressed CD19+ and CD20+ antigens decreased after 6 weeks of treatment with BT with statistical significance, p=0.014 and p=0.08, respectively. At 6 months after completing the treatment, both the percentage and the absolute count of them continued to increase with statistical significance, p=0.012 and p=0.011, respectively.
Conclusion: BT has an immunomodulatory effect on B1a CD19+CD20+ and CD5+CD20+ lymphocytes in older Cuban adults during 6 weeks of treatment.
B1a lymphocytes; Flow cytometry; Older adults; Immunosenescence
B1a lymphocytes were first described by Lee Herzenberg in the mouse [1,2]. These originate from a Lin-CD34+CD38lo hematopoietic stem cell [3]. In humans, they are located in the peritoneal and pleural cavities. They exhibit characteristics of activated lymphoid cells, being morphologically larger and with greater cytoplasmic complexity [1].
During fetal and neonatal life they predominate in the umbilical cord, where they represent between 60% and 80% of the total B cells and then decrease as the age of the individual advances [1,2].
They are part of the components of the innate immune response. They produce natural antibodies in a T-independent manner. They help the first line of defense against infectious microorganisms and constitute the only protection against encapsulated bacteria [4,5].
They act as a barrier immunity by favoring the change of isotype to immunoglobulin A, in the control of microorganisms on the cell surface at the level of the lymphoid tissue associated with the mucosa, they cross-react with epitopes expressed on dead and aged cells and in this way, carry out a homeostatic activity by contributing to their elimination [6,7].
Griffin et al. reported a new phenotype of B1a lymphocytes in cells from peripheral blood and umbilical cord blood. This consisted of the coexpression of CD20+CD27+CD43+ antigens, as well as the absence of CD70.70% of these lymphocytes also expressed the CD5 antigen [8].
B1a lymphocytes maintain their existence through a mechanism of self-renewal of pre-existing cells, a process that declines with age, a phenomenon known as immunosenescence. In this period of life, infections and chronic non-communicable diseases are more common [9].
Immunosenescence is a flexible process, that is, it can be modified by nutritional and pharmacological interventions. Different components of innate and adaptive immunity can be renewed and activated with the use of cytokines and other products with immunomodulatory action [10].
Biomodulina T® (BT) is a Cuban product from the Biopreparations Center (BioCen) registered in 1994 for the treatment of recurrent infections in older adults. Is a biological immunomodulator of natural origin, not derived from blood and composed of specific fractions of natural bovine thymus polypeptides [11].
Different researchers report the benefits of using this product in both children and adults to reduce the frequency and severity of infections [12,13].
BT is also indicated in pediatric patients with thymus hypoplasia, chronic obstructive pulmonary disease, as well as in the restoration of the immune system of long-lived patients with cancer [14,15]. Recently, BT demonstrated its safety and effectiveness in older adults, during the COVID-19 epidemic in Cuba [16].
The objective of this research was to determine the effect induced by BT on B1a lymphocytes in older Cuban adults.
Design and subjects
This research resulted from a clinical trial titled: “Evaluation of the efficacy and safety of a new dosage regimen of BIOMODULINE T® for the prevention of infections, including COVID-19, in older adults in Cuba”, who’s Promoting Center was the BioCen and as the coordinating Center, the National Clinical Trials Coordinating Center (CENCEC), with public registry.
A cross-sectional study was carried out in March 2020. The sample consisted of 30 older adults, aged between 60 and 99 years, from the “Alfredo Gómez Gendra” Nursing Home, in Havana.
Inclusion criteria
Older adults aged 60 years and older, of any sex and skin color, who expressed written consent to participate in the study.
Exclusion criteria
Older adults who had received BT in the last two months, with hypersensitivity to any component of the formulation, acute allergic states or history of severe allergic reactions, with uncontrolled intercurrent illness that included: Acute infections with concomitant fever, symptomatic heart failure, angina unstable chest and immunosuppressive treatment.
Treatment
Subjects included in the study received 1 bulb of BT intramuscularly, twice a week, for six weeks.
Obtaining and procedure of the biological sample
From all subjects, 3 ml of peripheral blood was extracted in tubes (Vacutainer®), with Ethylene Diaminetetraacetic Acid (EDTA) before treatment with BT®, one week after the end of the treatment (7th week) and six months later. The determination of B1a lymphocyte subpopulations (CD19+CD5+, CD20+CD5+ and CD19+CD20+) was performed by flow cytometry, in the Department of Immunochemistry and Immunology of the Institute of Hematology and Immunology, Havana, Cuba. 50 μL of whole blood was added to 15 mL tubes. Subsequently, 5 μL of the monoclonal antibodies conjugated to fluorochromes were added: Anti-CD20FITC, anti-CD5PE, anti-CD19APC, (DaKo Multi MixTM) and anti-CD45PerCP (Miltenyi Biotec), which were incubated at Room Temperature (RT). And protected from light, for 20 min. The red blood cells were then lysed with lysing solution for 10 min at RT. The cells were washed twice with 0.9% sodium chloride and centrifuged for 10 min, at 1500 rpm.
The reading of the samples was carried out on a Gallios, Beckman Coulter flow cytometer. 100,000 cells per tube were acquired. Data analysis was carried out with Kaluza Analysis® software version 1.2.
The different populations of B1a lymphocytes were evaluated and compared with the previously established reference value ≤ 12% for CD19+CD5+ and CD20+CD5+ B lymphocytes, respectively and 5-15% for CD19+CD20+ [17].
For the cellular analysis strategy, the dot plot of the total number of labeled cells was used to separate the singlets; cells that pass through the laser only once and present uniform signal height (FSC-H) and signal area (FSC-A) and that the cytometer registers as an event. In this way, cellular aggregates (two or more cells) are discarded. Next, the SSC-A vs. CD45PerCP-A dot plot was analyzed to separate the lymphocyte population on the previously chosen singlets. Three other dot plots were run from the CD45+ lymphocyte population (Figure 1).
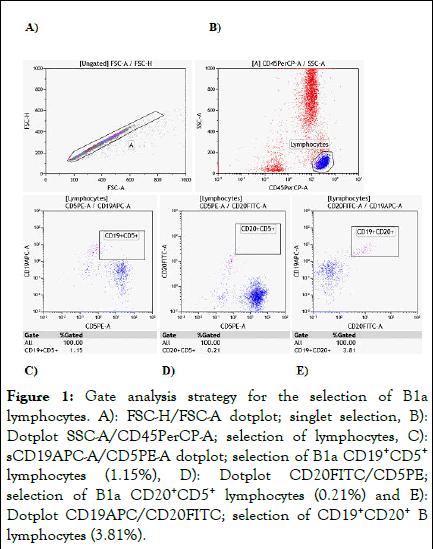
Figure 1: Gate analysis strategy for the selection of B1a lymphocytes. A): FSC-H/FSC-A dotplot; singlet selection, B): Dotplot SSC-A/CD45PerCP-A; selection of lymphocytes, C): sCD19APC-A/CD5PE-A dotplot; selection of B1a CD19+CD5+ lymphocytes (1.15%), D): Dotplot CD20FITC/CD5PE; selection of B1a CD20+CD5+ lymphocytes (0.21%) and E): Dotplot CD19APC/CD20FITC; selection of CD19+CD20+ B lymphocytes (3.81%).
Statistical processing
A database was created using Microsoft Excel with the data collected from the counts and percentages of CD5+CD19+, CD5+CD20+ and CD19+CD20+ B1a lymphocytes. Ranges of reference values obtained in studies carried out on healthy Cuban adults were used [17]. The Shapiro-Wilk test was used to evaluate the normality of the distribution of the variable values. Descriptive statistics were evaluated: Median, Standard Deviation (SD) and Odds Ratios (OR) before treatment with BT ®, after six weeks and six months after completing it. The significance threshold was set at p ≤ 0.05. The data were processed using the GraphPad Prism statistical program version 9.5.0.
The study was carried out in accordance with the provisions of the Declaration of Helsinki of the World Medical Association, with the last update at the 64th General Assembly, Brazil, 2013 [18].
The protocol was reviewed and approved by the Research Ethics Committee of the Center for Research on Longevity, Aging and Health (CITED). There was no conflict of interest between the researchers. The confidentiality of the results obtained was complied with.
The analysis of the percentage of B1a CD5+CD19+ lymphocytes showed a slight decrease in the median (5,480 vs. 4,830, SD 7,773 vs. 4,240) after six weeks of treatment with BT®, while that of the absolute count increased (57.87 vs. 79.78, SD 193.7 vs. 70.91), without statistical significance. For their part, both the percentage and their absolute count six months after the end of the treatment exhibited values greater than the medians (5.480 vs. 8.380, SD 7.773 vs. 7.575) and (57.87 vs. 139.9, SD 193.7 vs. 128.5) respectively, although without reaching statistical significance.
The exploration of B1a CD5+CD20+ lymphocytes revealed an increase in the medians of both the percentage and the absolute count, after six weeks of treatment with BT® (0.955 vs. 2.110, SD 6.017 vs. 2.380) and (17.02 vs. 40.58, SD 80.09 vs. 39.70), with statistical significance, p=0.026 and p=0.014, respectively. These two parameters remained elevated six months after finishing the course (0.955 vs. 2.120, SD 6.017 vs. 4.277) and (17.02 vs. 38.91, SD 80.09 vs. 62.86), without statistical significance [19].
The percentage and absolute count of lymphocytes that coexpressed CD19+ and CD20+ antigens decreased after six weeks of treatment with BT® (4.865 vs. 1.935, SD 4.692 vs. 4.566) and (88.48 vs. 31.03, SD 62.14 vs. 65.54), with statistical significance. p=0.014 and p=0.08, respectively. Six months after completing the treatment, both the percentage and the absolute count of them remained increased (4.865 vs. 8.465, SD 4.692 vs. 26.69) and (88.48 vs. 173.5, SD 62.14 vs. 323.8), with statistical significance, p=0.012 and p=0.011, respectively (Table 1).
| Immunophenotypes (%) | Timeline | Median ± SD before and after treatment | OR (95% CI) | p |
| CD5+CD19+ | 6 weeks post-treatment | 5.480 ± 7.773/4.830 ± 4.240 | 0.96 (0.88;1.04) | 0.976 |
| (≤12%) | 6 months post-treatment | 5.480 ± 7.773/8.380 ± 7.575 | 1.05 (0.97;1.12) | 0.09 |
| CD5+CD19+ | 6 weeks post-treatment | 57.87 ± 193.7/79.78 ± 70.91 | 1.00 (0.99;1.00) | 0.701 |
| [-] (cells per L) | 6 months post-treatment | 57.87 ± 193.7/139.9 ± 128.5 | 1.00 (1.00;1.00) | 0.121 |
| CD5+CD20+ | 6 weeks post-treatment | 0.955 ± 6.017/2.110 ± 2.380 | 0.97 (0.87;1.09) | 0.026* |
| (≤12%) | 6 months post-treatment | 0.955 ± 6.017/2.120 ± 4.277 | 1.02 (0.92;1.12) | 0.131 |
| CD5+CD20+ | 6 weeks post-treatment | 17.02 ± 80.09/40.58 ± 39.70 | 1.00 (0.99;1.01) | 0.014* |
| CD19+CD20+ | 6 weeks post-treatment | 4.865 ± 4.692/1.935 ± 4.566 | 0.91 (0.81;1.02) | 0.014* |
| [-] (%) | 6 months post-treatment | 4.865 ± 4.692/8.465 ± 26.69 | 1.10 (1.02;1.18) | 0.012* |
| CD19+CD20+ | 6 weeks post-treatment | 88.48 ± 62.14/31.03 ± 65.54 | 0.99 (0.98;1.00) | 0.018* |
| [-] (cells per L) | 6 months post-treatment | 88.48 ± 62.14/173.5 ± 323.8 | 1.01 (1.00;1.01) | 0.011* |
Table 1: Distribution of B1a lymphocytes in older adults before and after treatment with BT®.
As the individual's life progresses, changes occur in both the composition and function of the components of the immune system. The greatest evidence of the effect of age on the B compartment is the dramatic decrease in circulating B lymphocytes [10,19,20].
Different researchers reported a decrease in B1a CD5+CD19+ and CD5+CD20+ lymphocytes in older adults, especially in those over 80 years of age [10,20]. Older adults are more susceptible to recurrent infections, neurodegenerative, cardiovascular and neoplastic diseases of mature B cells, such as: small lymphocyte, diffuse large cell and mantle lymphomas [20].
A deficiency in the number of CD5+CD19+ B lymphocytes has also been found in the peripheral blood of patients with inflammatory bowel diseases, including: Ulcerative colitis and Crohn's disease. In these patients, an inverse correlation has been described between the number of these lymphocytes and the activity of the disease, which suggests a loss of immunological tolerance to the antigens of the intestinal mucosa, which is greater in the elderly. More advanced. B1a lymphocytes can act in a manner similar to regulatory B cells, as they produce high concentrations of anti-inflammatory cytokines, such as: Interleukins -3, -10, and -35.
In a previous work, elderly Cubans aged 80 years or older exhibited lower absolute counts of CD19+CD20+ B lymphocytes.
The decrease in circulating B cells in peripheral blood in older adults is primarily due to a depression in the formation of new cells in the bone marrow, which affects lymphopoiesis. This reduction of naïve B cells is accompanied by an expansion of memory B cells, with defects in their ability to differentiate into plasma cells, characteristics of an immunosenescence phenotype, related to the development of diseases, as the age of the patient advances individual [20].
Treatment with BT has proven to be effective in older Cuban adults with recurrent infections [16]. The effect has been highly significant in increasing the percentages of total CD19+ B lymphocytes. Ramos HE and collaborators demonstrated in elderly people treated with BT and VAMENGOC-BC® vaccine (individual and combined) that BT significantly increased the expression of the CD19+ antigen. Hernández and collaborators found that BT increased the percentages and absolute counts of total CD19+ B lymphocytes six weeks after completing the treatment, in older Cuban adults, an effect that was maintained for six months later [16]. This result contributed to the intervention that was developed in Cuba with the administration of BT in institutionalized Cuban elderly, during the COVID-19 epidemic, which decreased their morbidity and mortality due to SARS-CoV-2.
In this research, BT increased both the percentage and the absolute counts of B1a CD5+CD20+ lymphocytes six weeks after completing the treatment, an effect that showed a tendency to be maintained six months later, but without statistical significance. On the other hand, BT decreased both the percentage and the absolute count of B lymphocytes that coexpressed CD19+ and CD20+ antigens after six weeks of treatment. However, six months after completing its administration, these two parameters increased again, with figures higher than the percentages and absolute counts presented before treatment.
This result suggests that BT has a justified complex immunomodulatory effect, firstly, inducing CD5+CD20+ B1a with a negative effect on CD19+CD20+ and in the long term (up to 6 months) increasing the latter. From a clinical point of view, this effect can translate into better immunity against infections and at the same time, less tendency to inflammatory responses, taking into account the regulatory role of B1a lymphocytes. The results coincide with previous findings (15,16) in terms of duration of the response induced on B and T lymphocytes in older adults up to at least 6 months, hence the need to administer new treatment cycles, according to the need of each individual.
BT has an immunomodulatory effect on B1a CD19+CD20+ and CD5+CD20+ lymphocytes in older Cuban adults and partially maintains its effect up to six months after completing the treatment, which suggests its cyclical application in this group of vulnerable individuals, to prevent and treat infections and autoimmune and chronic B lymphoproliferative diseases, involving B1a lymphocytes.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Suarez VM, Hernandez IC, Ramos EH, Marrero YT, Perez YD, Garcia MS, et al. (2025) Immunomodulatory Effect of Biomodulin T® on B1a Lymphocytes in Older Cuban Adults. J Clin Cell Immunol. 16:753.
Received: 13-May-2024, Manuscript No. JCCI-24-31346; Editor assigned: 16-May-2024, Pre QC No. JCCI-24-31346 (PQ); Reviewed: 30-May-2024, QC No. JCCI-24-31346; Revised: 05-Mar-2025, Manuscript No. JCCI-24-31346 (R); Published: 12-Mar-2025 , DOI: 10.35248/2155-9899.25.16.753
Copyright: © 2025 Suarez VM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.