
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2022)Volume 13, Issue 5
Introduction: C-X-C motif chemokine receptor 4 (CXCR4) plays a prominent role in inflammation, atherosclerosis, and cancer biology. Therefore, CXCR4 represents a promising target for molecular imaging in cardiovascular diseases such as atherosclerosis and arterial wall injury.CXCR4 and its cognate ligand, stromal cell–derived factor 1α (SDF- 1α), induced monocyte recruitment to the injured endothelium and subsequent plaque formation is the crucial progression of atherosclerosis. CXCR4 was been proved to be intensively expressed on monocytes/macrophage. [68Ga]-APD, based on CXCR4 antagonists TIQ-15, had designed as a PET tracer for imaging atherosclerosis. The aim of this study was to evaluate the biological characteristics of [68Ga]-APD, and compared with [18F]-FDG, [18F]- NaF, and [68Ga]-Pentixafor.
Results: The specification and quality of APD was identified by Mass, NMR and HPLC. After being labeled with Ga-68 under acetate buffer (pH=5.5), radiochemical purity was over 90% and stable for more than 4 hours in 37°C human serum. After being injected from tail vein on apolipoprotein-E-deficient (ApoE-/-) atherosclerotic mice, hydrophilic [68Ga]-APD was quickly eliminate from the kidney and bladder and accumulate in the atherosclerotic aorta. The highest target/background ratio (TBR) on atherosclerotic sites were 17.68 ± 0.71 (n=3) on high-fat diet ApoE-/- mice for 12 weeks after [68Ga]-APD injection about 1 hour. However, the TBR of [68Ga]-Pentixafor was only 2.06 ± 0.67 (n=3) on the same mice model. Competitive study represented that CXCR4 antagonist AMD3465 could effectively block the uptake of [68Ga]-APD on the atherosclerotic site and CXCR4 expression organs. Comparing with [18F]-FDG and [18F]-NaF, [68Ga]-APD represented relatively better TBR and specificity on the imaging of atherosclerotic lesions.
Conclusion: In vivo evaluation of CXCR4 expression in ApoE-/- mice revealed the high TBR of [68Ga]-APD on the atherosclerotic aorta and better than [68Ga]-Pentixafor. These evidences represented its feasible as a surrogate marker for inflammatory atherosclerosis.
Autism spectrum disorders; Cytokine; Monocytes; microRNA (miRNA)
Although the diet management and medical improvement in treating Cardio Vascular Diseases (CVD), atherosclerotic plaque- induced stroke and Coronary Heart Disease (CHD), heart attack, or angina are one of the leading causes of mortality and morbidity worldwide [1,2]. The pathogenesis of atherosclerosis is characterized by the chronic accumulation of lipids and pro-inflammatory immune cells to the focal arterial wall and progressively narrows the lumen of the artery [3-5]. Owing to the unstable plaques by the pathologic mechanisms of inflammation, atherosclerotic plaque formation can be diagnosis from the mechanism of inflammation [6,7]. Within the inflammation and different aspects of the atherosclerotic plaque, activated macrophages play the most interesting target in the atherosclerotic lesion [8]. Imaging atherosclerosis with PET/CT characterizes atherosclerotic plaques on a molecular level and enables the quantification of arterial calcification [9]. Many Functional PET imaging tracers have been tried to targeting molecular elements of atherosclerosis of increasing the glucose metabolism by the inflammatory cells [10-13] and detect micro-calcifications around the arterial wall [14-16], such as [18F]-FDG and [18F]-NaF, respectively. The 7-transmembrane helix G-protein–coupled receptor, Chemokine C-X-C motif receptor 4 (CXCR4), and its cognate ligand, stromal cell-derived factor 1a (SDF-1a/CXCL12), induced monocyte recruitment to the injured endothelium and subsequent plaque formation is the crucial progression of atherosclerosis. CXCR4 is also been proven to be intensively expressed on monocytes/ macrophage [17-21]. Recently, CXCR4-directed of macrophages PET imaging with [68Ga]-radiolabeled cyclic peptide, Pentixafor, has been provided to imaging neoplasms, hematologic, stroke, atherosclerosis, and myocardial infarction in humans by the mechanism of overexpression CXCR4 [22-25]. In addition, Tetra- azamacrocycles based small-molecule CXCR4 antagonists, such as AMD3100 and AMD3465, are also developed and proven to bind on human and mouse variants CXCR4 receptor. Jacobson et al., had developed a one-step radiosynthesis of [64Cu]-AMD3100 and exhibited a promising agent for visualization of CXCR4- positive tumors and metastases [26]. However, Copper (II) cyclam complexes are unlikely to have sufficient electrostatic/H-bonding between cyclam amine groups with aspartate residue side chains (171 and 262) and lead to high nonspecific hepatic accumulation to retain the metal ion in vivo [27].
The atherosclerotic-prone apolipoprotein E-deficient (ApoE-/-) mice were first developed in 1992 by homologous recombination of embryonic stem cells and currently use in the pre-clinical atherosclerotic model by down regulation of cholesterol transporter ATP-binding cassette subfamily A member 1 levels [28]. Poor lipoprotein clearance of atherosclerosis-prone apolipoprotein E-deficient (ApoE-/-) mice display the accumulation of cholesterol ester-enriched particles in the blood and promote the development of atherosclerotic plaques. ApoE-/- mice is well established for studying atherosclerotic lesions on aorta by high-fat chow diet or even a regular diet for several weeks. The atherosclerotic lesions in ApoE-/- mice resemble human lesions in their predilection sites and progression to the fibro-proliferative stage [29]. In this study, we investigate the biological characteristics of novel CXCR4 agent [68Ga]-APD, and compared with [18F]-FDG, [18F]-NaF, and [68Ga]-Pentixafor PET tracers on targeting atherosclerotic lesion by high fat diet ApoE-/- mice model.
General
APD structure was designed and selected from Tetrahydroisoquinoline-Based CXCR4 Antagonist TIQ-15 by screening million’s chemical fragments and evaluated its chemical binding energy on CXCR4 specific amino acids residue [30]. APD was synthesized at RDD LAB. INC, Taiwan and determined its specifications by NMR (JNM-ECZ500R/S1, JOEL USA, Inc), Mass (AB Sciex (Concord, ON, Canada) 4000QTRAP® mass spectrometry) and high-performance liquid chromatography (HPLC, Waters-2695, with UV and radio detector). Radio-thin-layer chromatography was performed on instant TLC chromatogram sheets (Sigma Chemical Company, USA), eluting with citrate buffer (pH=5.5) and Bioscan AR-2000 (Eckert and Ziegler, Germany). Pentixafor was purchased from UNION CHEMICAL IND. CO., LTD. Taiwan. [18F]-FDG and [18F]-NaF provided from Institute of Nuclear Energy Research, Taoyuan, Taiwan (Figure 1).
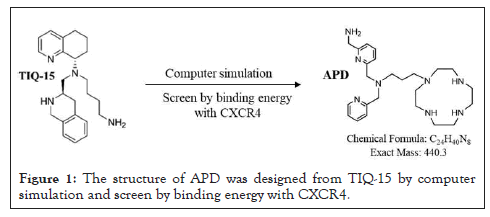
Figure 1: The structure of APD was designed from TIQ-15 by computer simulation and screen by binding energy with CXCR4.
Preparation and radiochemical purity (RCP) analysis
APD (0.01~0.1 mg) dissolved in acetate buffer (pH 5.0~6.0) and then labeled with [68Ga]-GaCl3 (1~10mCi) for at least 20 minutes under 60~70℃.To determine the amount of impurity, the sample was chromatographed on ITLC-SG (Sigma Chemical Company, USA) using 0.1 M citrate buffer (pH 5.5) as the mobile phase (Rf=0: [68Ga]-APD, Rf=1: [68Ga]-GaCl3). [68Ga]-colloid was also determined by TLC with 1.25 M NH4Ac, pH 5.5: DMF (1:1) as a mobile phase (Rf=0: [68Ga]-colloid, Rf=1: [68Ga]-APD) [31].
A Waters system pump (Germering, D) and radiometric detection (Bioscan, Washington DC), were used for RP-HPLC analysis. C-18 column (Waters 5 μm, 80 Å, 250 × 4.6 mm). Flow rates of 0.8 mL/min were employed as the following: A linear gradient eluent starting from 30% A (0.1% TFA in water) and 70% B (0.1% TFA in acetonitrile), increasing to 90% B for 10 min, remaining constant at 90% B for another 15-30 min. The RCP was calculated expressing the peak corresponding to [68Ga]-APD as percentage of the total activity in the radio-chromatogram. The injected activity on the HPLC-column was in the order of 5 MBq. RCP of [68Ga]‐ Pentixafor was also determined by HPLC followed as previously described [32,33].
Study product compliance
Compliance was calculated based on dispensed and returned study products. In the case of missing products or no return, compliance was estimated from subject diaries.
Partition coefficient (n-Octanol/PBS) determination
To determine the hydrophilicity of [68Ga] ‐APD, the octanol/water partition coefficients (log P) were measured. Briefly, [68Ga]-APD (7.4 MBq) with phosphate-buffered saline (PBS, 5.0 mL, 0.15 M, pH 7.4) and n-octanol (5 mL) within a test tube. After vortexing for 1 min, tube was placed on bench for 3 min in room temperature. Samples (from each phase, 100 μL) were counted its radioactivity by using gamma-counter. The tests were reported as mean ± SD (n ≧ 3), independently.
Mice and PET/CT acquisition
ApoE-/- mice experimental protocol had been approved by the Institutional Animal Care and Use Committee (IACUC) in the Institute of Nuclear Energy Research (Taoyuan, Taiwan) and followed the ARRIVE guidelines and the USPHS Policy on Human Care and Use of Laboratory Animals. Female ApoE-/- mice (provided from Taiwan University Hospital) (aged >8 weeks) housed in a controlled environment (22 ± 2°C and 50 ± 5% relative humidity) with a 12-h light-and-dark cycle and received a high-fat diet for accelerating atherosclerotic lesions. PET/CT imaging was performed on a Bioscan scanner (matrix size, 128 × 128 × 159; CT attenuation-corrected; non-scatter corrected) (Washington DC, USA) following a intravenous tail vein injection of approximately 14.8 MBq (0.4 mCi) of [68Ga] ‐APD into ApoE-/- mice (3-5 mice per group). Dynamic scans were acquired in list mode format for at least 120 min, and sorted into 22 frames, 0.5-mm sinogram bins for image reconstruction (4 × 15 s, 4 × 60 s, 11 × 300 s, 3 × 600 s). Mice were anesthetized with isoflurane (3% for induction and 2% for maintenance) throughout the experiment.
ApoE-/- mice was imaged with [68Ga]‐APD (11.1 MBq) on 2, 8 and 12 weeks of high-fat diet to characterize different severity on atherosclerotic lesions. For competitive inhibition assay on [68Ga]‐ APD, a CXCR4 inhibitor AMD3465 (25 mg/kg) was injected before administration of 11.1 MBq of [68Ga]‐APD on high-fat diet ApoE-/- mice for 12 weeks. [68Ga]‐ Pentixafor (11.1 MBq) was also administrated on the same mice model for comparing the efficacy on atherosclerotic lesions with [68Ga]‐APD. [18F]- FDG PET/CT imaging was been performed after 6 h fasting period to ensure serum glucose levels below 130 mg/dL. To estimate the radioactivity concentration, volumes of interests had defined on coregistered PET/CT images using PMOD software. Target/ Background Ratio (TBR) had calculated by placing a circular 2-mm volume of all interest organs/tissues around a site. TBRs were been calculated as focal uptake divided by blood pool.
Autoradiographic studies on atherosclerosis
To visualize the distribution of [68Ga]‐APD around the heart region, autoradiography was performed using 20-μm-thick fresh-frozen heart and aorta sections derived from ApoE-/- mice. The specimens were exposed to phosphor imaging plates (MS-2040, Fuji Photo Film Co. Ltd.) for 2 h in the [68Ga]‐APD examination. Subsequently, the radioactivity distributions were imaged by using imaging analyzer BAS 4000 (Fuji Photo Film Co. Ltd.).
Statistical analysis
The statistical significance of comparisons of radioactivity in organs in PET studies was based on 2-sided, 2-sample Student t-tests. For all tests, P<0.05 was considered statistically significance and represented on figures. Results were reported as mean ± SD (n ≧ 3).
Synthesis and characterization
The novel CXCR4 antagonist structure of APD was represented (Figure 1). The binding energy of APD and TIQ-15 were -300.3 kcal/mol and -80.4 kJ/mol, respectively. Chemical characterization of APD had verified by NMR, mass spectrum and HPLC. 1H NMR (D2O) δ8.46-8.45 (m, 1 H), 7.84-7.80 (m, 2.H), 7.49-7.47 (d, 1H), 7.41-7.34(m, 3H), 3.70-3.90 (m, 6H),2.78-2.47 (m, 20H), 1.73-1.67 (m, 2H). 13C NMR (D2O) δ160.30, 157.53, 148.16, 138.61, 137.96, 124.63, 123.08, 122.84, 120.45, 59.98, 53.29, 50.30, 45.93, 45.10, 43.92, 43.29, 23.31. The molecular weight and purity of APD was measured as [M+H]+=441.51 and over 98%, respectively.
Radiochemical purity (RCP) and partition coefficient (n-Octanol/PBS) determination
After the labeled of APD with [68Ga]-GaCl3 under 60~70℃ for 20 minutes, radiochemical purity of [68Ga]-APD was calculated by paper chromatography on SG-ITLC strip with citrate buffer (pH 5.5) (Figure 2A). The chromatogram represented that the radiochemical purity of [68Ga]-APD was over 90%. [68Ga]-colloid was excluded as the impurity by the determination of TLC with 1.25 M NH4Ac, pH 5.5: DMF (1:1) as a mobile phase.
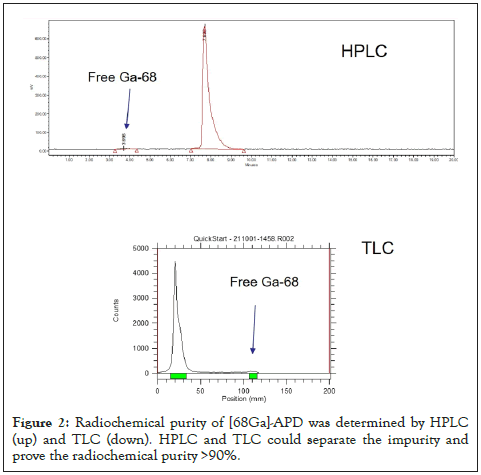
Figure 2: Radiochemical purity of [68Ga]-APD was determined by HPLC (up) and TLC (down). HPLC and TLC could separate the impurity and prove the radiochemical purity >90%.
PET/CT studies on ApoE-/- atherosclerotic model mice
In the competitive inhibition study, the PET/CT images represented that [68Ga]-APD could be apparently blocked the uptake on atherosclerotic lesion by pre-injection of a dose (5 mg/ kg) of CXCR4 inhibitor AMD3465 on high fat diet ApoE-/- mice for 12 weeks. Furthermore, the pre-dosing with AMD-3465 could also significantly reduce the TBR of [68Ga]-APD at CXCR4- overexpression organs, such as liver, lung, and kidney (27). These evidences provided that [68Ga]-APD was specific binding on CXCR4 (P<0.05) (Figure 3).
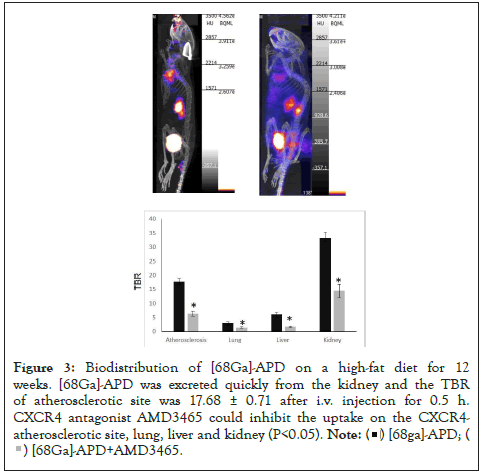
Figure 3: Biodistribution of [68Ga]-APD on a high-fat diet for 12 weeks. [68Ga]-APD was excreted quickly from the kidney and the TBR of atherosclerotic site was 17.68 ± 0.71 after i.v. injection for 0.5 h. CXCR4 antagonist AMD3465 could inhibit the uptake on the CXCR4-atherosclerotic site, lung, liver and kidney (P<0.05).
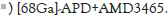
In comparing the PET images of [68Ga]-APD (11.1 MBq) on the different duration ApoE-/- mice, the TBR was 7.52 ± 0.83, 9.65 ± 0.69 and 17.68 ± 0.71 on 2, 8 and 12 weeks of high fat diet, respectively. The TBR of [68Ga]-APD was decreased with time (n=3) (Figure 4).
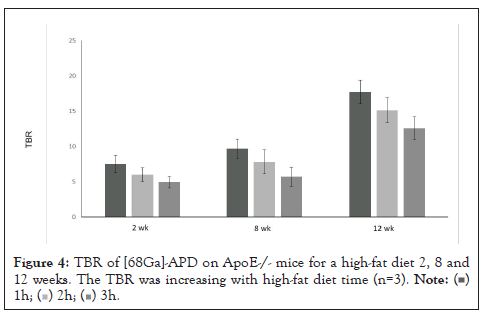
Figure 4: TBR of [68Ga]-APD on ApoE-/- mice for a high-fat diet 2, 8 and 12 weeks. The TBR was increasing with high-fat diet time (n=3). 
Kidney and bladder had higher tracer uptake, providing that [68Ga]-APD might primarily excrete from the kidney to bladder. For comparing the imaging efficacy on atherosclerotic aorta between [68Ga]-APD and [68Ga]-Pentixafor (RCP>95%) on high- fat diet ApoE-/- mice for 12 weeks, the results represented that TBR of [68Ga]-APD was better than [68Ga]-Pentixafor during the imaging time (Figure 5).
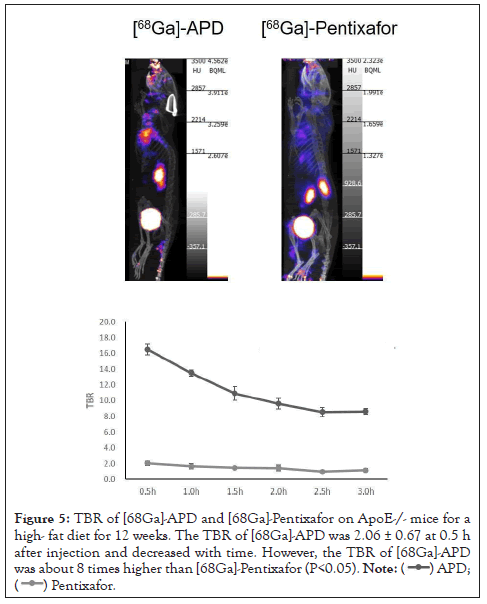
Figure 5: TBR of [68Ga]-APD and [68Ga]-Pentixafor on ApoE-/- mice for a high- fat diet for 12 weeks. The TBR of [68Ga]-APD was 2.06 ± 0.67 at 0.5 h after injection and decreased with time. However, the TBR of [68Ga]-APD was about 8 times higher than [68Ga]-Pentixafor (P<0.05).
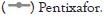
The TBR on [68Ga]-Pentixafor was approximately matched as previously described [22-25]. [18F]-FDG injection was been performed after fasting for at least 6 h to ensure serum glucose levels below 130 mg/dL. The PET image represents that [18F]-FDG accumulated mainly on the myocardium. However, the highest TBR of [18F]-FDG was only 1.53 ± 0.75 (n=3) on the atherosclerotic aorta (ApoE-/- mice, high-fat diet 10 weeks). Furthermore, [18F]- NaF could not effectively target atherosclerotic site by the reason of lacking calcified lesions on this model (ApoE-/- mice, high-fat diet 10 weeks) (Figure 6).
Figure 6: TBR of [68Ga]-APD, [18F]-FDG and [18F]-NaF on ApoE-/-mice high fat diet for 10 weeks. [18F]-FDG was major distributed in the myocardium. [68Ga]-APD distributed specifically in the atherosclerotic site. [18F]-NaF could not find to target on heart and atherosclerotic site.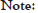
Autoradiographic studies on atherosclerosis
From the PET/CT image of [68Ga]-APD in Figure 6, there was tracer specific targeting in the chest region. For verified the distribution of [68Ga]-APD, the slide autoradiogram of [68Ga]- APD had shown obvious high radioactivity accumulating in the aorta region but not on the heart. Oil Red O stained tissues had also proved the distribution of lipids on the atherosclerotic aorta (Figure 7).
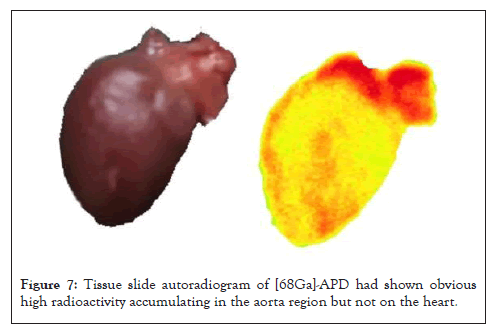
Figure 7: Tissue slide autoradiogram of [68Ga]-APD had shown obvious high radioactivity accumulating in the aorta region but not on the heart.
This study reports a novel CXCR4 antagonist structure APD was been designed by replacing over million’s chemical fragments on the structure of TIQ-15 and evaluating its binding energy. After being labeled with Ga-68, [68Ga]-APD represent good sensitivity and specificity to CXCR4 expression atherosclerotic lesions.
Atherosclerotic plaque formation, development, and progression are complex and involve many factors, including endothelial dysfunction, elevated cholesterol and immune activity. As plaques in the arterial walls build and progress, the rupture on the arteries may be occluded distally and block blood flow into heart muscle, brain, and other parts of the body [1]. Inflammation is an important component of atherosclerotic plaque vulnerability. Owing to the glucose uptake in active and lipid-rich macrophages on inflammatory plaques, it is likely to use [18F]-FDG as a marker to detect small lesions of atherosclerotic plaques. However, [18F]- FDG plaque imaging is limited by non-specificity on atherosclerotic lesions. In addition, the constant motion of cardiac cycle and the significant uptake of [18F]-FDG in the myocardium has decreased the enthusiasm to use this tracer in atherosclerotic imaging [25]. [18F]-NaF have significant advantages in the assessment of atherosclerotic plaques imaging with regards to its more accurately and precisely in detecting and characterizing plaques than [18F]- FDG. Therefore, the future of PET imaging to assess atherosclerosis will predict to heavily rely on [18F]-NaF [34].
Oxidized low-density lipoprotein (Ox-LDL) and vascular hypoxic process might significantly induce macrophage to overexpression CXCR4 and enhanced the atherosclerotic progression. In comparison within early or advanced atherosclerotic lesions, Bot et al. had found that CXCR4 expression was significantly pronounced in advanced unstable lesions [35]. Remarkably, the expression of CXCR4 protein was co-localized with macrophage expression in inflamed vulnerable plaque lesions. This finding might support the concept that CXCR4 played a recruitment role for leukocytes into the vessel wall and could serve as a good biomarker for atherosclerotic imaging [27].
There are many PET tracers have tried to image atherosclerotic changes based on the macrophage to expression CXCR4. [68Ga]- Pentixafor is one of the successful CXCR4 clinical trial tracers to image atherosclerosis with a clear view of vascular uptake and a relatively clear background. Li et al. had shown that patients with higher uptake of [68Ga]-Pentixafor represented a higher incidence of metabolic syndrome and might suggest its clinical potential in the evaluation of diseased vessels [36]. Derlin et al. had also shown that accumulation of [68Ga]-Pentixafor and [18F]-FDG represented only a weak correlation by observed in non-calcified lesions [37].
Tetrahydroisoquinoline-Based CXCR4 antagonists TIQ-15 had been found to have good oral bioavailability and its active structure was confirmed to interaction with ASP 97, Tyr116 and Glu288 residue on CXCR4 [38]. In this study, a novel CXCR4 tracer APD was been designed from the structure of TIQ-15 to become a tracer for imaging atherosclerosis. The selected structure of APD was been screened by replacing over million’s chemical fragments on the structure of TIQ-15 and evaluating its docking efficacy by binding energy. In our preliminary study, the cellular uptake of [68Ga]-APD could be blocked by using CXCR4 antagonist AMD3465 on CXCR4-expression HT-29 colon cells. The PET image of [68Ga]-APD on ApoE-/- atherosclerotic model had shown high selective uptake on atherosclerotic aorta. Owing to the good hydrophilicity (Log P=-1.4), [68Ga]-APD PET image showed a quick excretion from kidney and with a relatively low background. CXCR4 antagonist AMD3465 (5 mg/kg) could effectively reduce the uptake on atherosclerotic plaque, liver, lung and kidney at 60 min before tracer administration which suggested its specific binding on CXCR4 in Figure 3. Human serum stability assays also showed that [68Ga]-APD could be stable for over 4 h under 37℃ human serum. For confirming the distribution of isotope labeled APD on the atherosclerotic aorta, tissue frozen section had also been done after the bio-distributed of [68Ga]-APD (RCP>90%) for 30 mins on ApoE-/- mice. The autoradiogram represented that [68Ga]-APD was distributed on atherosclerotic aorta which can be seen in Figure 7. Atherosclerotic plaques may stabilize or reduce in size, lipid content, foam cell content, and macrophage inflammation through Macrophage Reverse Cholesterol Transport (RCT) mechanism by eliminating oxidized low-density lipoprotein (Ox-LDL) from atherosclerotic plaques. [39,40]. Early detection and therapy is the key to improving the atherosclerotic prognosis at present. In this study, [68Ga]-APD PET image provides the possibility in the early diagnosis of atherosclerosis with high sensitivity and specificity than [68Ga]-Pentixafor.
In vivo evaluation of CXCR4 expression in ApoE-/- mice revealed the high TBR of [68Ga]-APD on the atherosclerotic aorta and better than [68Ga]-Pentixafor. These evidences represented its feasible as a surrogate marker for inflammatory atherosclerosis.
CH conducted experiments and wrote the manuscript. CY, CC and CP reviewed the study design and helped to cultured cells, raised mice and conducted mice’s experiments. All authors read and approved the final manuscript.
I would like to express my deep and sincere gratitude to research colleagues, Dr. Mei-Hui Wang, Dr. Hung-Wen Yu, Dr. Ying- Hsia Shih and all of the team members for his genuine support throughout this research work. I thank the management of Institute of Nuclear Energy Research for their financial support to do this work. I also thank the directors Dr. Shiou-Shiow Farn, Dr. Jeng- Hung Lee and Dr. Jeng-Jong Wang and Dr. Chih-Hsien Chang for their genuine support to complete this thesis successfully. Finally, my thanks go to all the people who have supported me to complete the research work directly or indirectly.
Ethics approval and consent to participate
ApoE-/- mice experimental protocol had approved by the Institutional Animal Care and Use Committee (IACUC) in the Institute of Nuclear Energy Research (Taoyuan, Taiwan) and followed the ARRIVE guidelines and the USPHS Policy on Human Care and Use of Laboratory Animals. ApoE-/- mice had gotten the consent to provide from Taiwan University Hospital.
Not applicable.
All the raw data of this study, including PET images and data can be obtained through the corresponding authors on reasonable request.
The authors declare that no other
Funding was all provided by Institute of Nuclear Energy Research, Taiwan.
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
[Crossref] [Google Scholar] [PubMed].
Citation: Hsia CC, Yeh CH, Chen CT, Peng CL (2022) Imaging the Cytokine Receptor CXCR4 in Atherosclerotic Plaques with [68Ga]-APD: A Novel Agent on Computer Simulation Approach. J Clin Cell Immunol.13:667.
Received: 06-Jun-2022, Manuscript No. JCCI-22-17763; Editor assigned: 09-Jun-2022, Pre QC No. JCCI-22-17763 (PQ); Reviewed: 23-Jun-2022, QC No. JCCI-22-17763; Revised: 30-Jun-2022, Manuscript No. JCCI-22-17763 (R); Published: 07-Jul-2022 , DOI: 10.35248/2155-9899.22.13.667
Copyright: © 2022 Hsia CC, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.