Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Research Article - (2021)Volume 9, Issue 7
Blood pressure lowering effect of Ficus deltoidea kunstleri (FDK) has been associated with changes in the Renin-Angiotensin-Aldosterone System (RAAS) and endothelial function in Spontaneously Hypertensive Rats (SHR). Whether it also involves other systems is unclear. This study therefore screened for other potential BP lowering mechanisms in FDK-treated SHR based on urine metabolite profile obtained using 1H-NMR spectrometry and multivariate data analysis. Male SHR were administered orally, either 0.5 mL of distilled water (control), or 1000 mg.kg-1 body weight of FDK or 10 mg.kg-1 body weight of losartan once daily for 4 weeks. BP was measured weekly and 24-hr urine was collected using metabolic chambers at the end of the 4th week. Animals were then euthanised and blood was collected for estimation of total anti-oxidant capacity TAC. Kidneys were harvested for antioxidant enzyme gene expression studies. BP in FDK- and losartan-treated SHR was significantly lower, whereas serum TAC was significantly higher than those in the controls. Orthogonal partial least square analysis indicated that FDK-treated SHR were separated from controls by the presence of a larger number of ketone body and amino acid metabolism intermediates. Superoxide dismutase-2, catalase and fork head box gene expressions were higher in kidneys of FDK-treated SHR than those in the controls. It appears that apart from RAAS, the BP lowering effect of standardized aqueous-ethanolic extract of leaves of FDK in SHR is also associated with increased TAC, antioxidant enzyme expressions and probably also involves multiple metabolic pathways, including those involving ketone bodies, amino acids and energy metabolism
Hypertension; Metabolomics; 1H-NMR spectroscopy; SHR; Ficus deltoidea kunstleri; Metabolomics
A wide variety of plants or herbs are used for medicinal purposes worldwide [1,2]. Ficus deltoidea is one of the many medicinal herbs that is popular amongst the Malays. It has long been used in post-natal care and to treat diseases such as diabetes and even high blood pressure [3]. Numerous laboratory studies suggest of its anti-diabetic, antioxidant and anti-inflammatory properties [4-6]. Although Ficus deltoidea has been traditionally used for the treatment of hypertension there are no scientific studies investigating its antihypertensive properties, except for our two recent studies on Ficus deltoidea angustifolia and Ficus deltoidea kunstleri in SHR [7,8]. Both of these herbs were found to significantly decrease blood pressure in a dose dependent manner. The antihypertensive effect was also associated with changes in concentrations and activities of some components of the Renin-Angiotensin-Aldosterone System (RAAS) and markers of endothelial activation.
Several chemical constituents of the seven varieties of FD have been reported and it is possible that some of these might be responsible for the blood pressure lowering property of FD. The chemical constituents include schaftoside, vicenin-3, 6-C-β-d-xylopyranosyl- 8-C-α-l arabinopyranosylapigenin and isoschaftoside in the FD varieties trengganuensis, intermedia and bilobata2; isovitexin, flavone with 1 hexose and 1 acetyl moieties, and vicenin-2 in FD var angustifolia and vitexin, flavone with 3 sugar moieties (hexose, rhamnose, arabinose) and orientin 2”-O-rhamnoside in FD var deltoidea, kunstleri, motleyana and bilobata 1 [9].
Although FDK has been shown to decrease blood pressure in SHR, and that this action might involve the RAAS and endothelial function, the exact mechanism underlying its antihypertensive effect, however, remains unclear. It is possible that the action may involve multiple mechanisms as flavonoids and polyphenols in plants are well known for their anti-oxidant activities. This study therefore attempted to identify the possible antihypertensive mechanism/s of FDK using metabolomics. For this, 1H-NMR- based metabolomic approach was used to identify differences in urinary metabolites between non-treated SHR controls and SHR treated with a standardized aqueous-ethanolic extract of leaves of FDK for four weeks.
All aspects of animal care and experimentation were approved by the Animal Care and Users Committee, Faculty of Medicine, University Technology Mara (UiTMCare 113/2015) as allowed under the Malaysian Animal Welfare Act 2015 (AWA 2015), along with the Animal (Amendment) Act 2013 (AA 2013). They were carried out in compliance with the ARRIVE guidelines 2.0 (July 2020). The Malaysian Code for care and use of animals for experimental purposes (My Code) is an adapted and adopted version (with permission from the National Health and Medical Research Council-Australia), of the Australian Code for the Care and Use of Animals for Scientific Purposes 7th and 8th Editions (NMHRC 2004 and NMHRC 2013 respectively). Only approved procedures were performed on the animals. Eighteen, healthy, male SHR aged 8-12 weeks, weighing 250-350 g were maintained under standard laboratory conditions of 12-h light and dark cycle with access ad libitum to food (Speciality feed, Australia) and water. The rats were acclimatized to the laboratory conditions and to the whole blood pressure measuring technique (including to the restrainers that were used during the measurement of blood pressure) for a period of seven days immediately prior to the start of the study. Restrainers were also left in their cages during the acclimatization period. The measurements were made at the same time of the day for each rat, in the same room, and by the same observer.
The plant sample of FDK was supplied by Atta-ur Rahman Institute of Natural Products Discovery (AuRIns), University Technology MARA (UiTM) Puncak Alam, Selangor, Malaysia. The plants were collected from Kuala Terengganu (a north- eastern state of Peninsula Malaysia), and were identified by Prof. Nashriyah Mat, a taxonomist from University Sultan Zainal Abidin. The specimen (voucher number of 00048) was deposited at the Herbarium of School of Agricultural Science and Biotechnology, Faculty of Bioresources and Food Industry in UniSZA. Losartan was purchased from MSD, UK.
Preparation of standardized ethanolic aqueous leaves extracts of FDK
The preparation of the extract was done at AuRIns, University Technology MARA (UiTM) Puncak Alam, Selangor, Malaysia. Fresh, FDK leaves were separated from the stems, cleaned with tissue paper and cut into small pieces that were then oven dried at 35°C for 3 days until completely dry. The dried leaves were then grounded into fine powder and sieved. For extraction, 100 g of the dried leaf powder was mixed with one liter of 50% ethanol/water solvent. The mixture was homogenized and sonicated for one hour at a temperature of below 40°C. Ice was used to control the temperature of the water bath and to ensure the temperature remained below 40°C. After this, the mixture was filtered twice using Whatman filter paper (grade 1) to ensure the complete removal of any debris. The extract was concentrated using vacuum rotary evaporator at 40°C and the samples were freeze dried to remove any remaining water in the extract. The crude extract was then transferred into an amber-coloured bottle and stored at 4°C. The FDK extract was diluted in distilled water before it was administered to the SHR
Study design
SHR were divided into three groups with six rats per group (n=6) and treated with either 0.5 ml of distilled water, or 0.5 ml of FDK extract (1000 mg.kg-1) or losartan (10 mg.kg-1 in 0.5 ml distilled water) [10]. In our earlier study investigating the effect of FDK on blood pressure we had used four different doses of FDK and noted that the best antihypertensive effect was obtained at a dose of 1000 mg.kg-1 body weight [11]. In this study we, therefore, used just one dose of FDK. In that study, losartan and captopril were also used as positive controls. The magnitude of decrease in blood pressure of a dose of 1000 mg.kg-1 of FDK was very similar to that of losartan and captopril. All treatments were given once daily for 4 weeks via oral gavage and the solutions for oral administration were prepared fresh each time. Animals were housed individually in metabolic cages during the period of study. Blood pressure was recorded weekly using tail-cuff plethysmography. On the last day of treatment, 24-hour urine samples were collected into sterile containers using metabolic cages. The urine was immediately stored at -80°C and was later used for metabolite analysis using 1H NMR. At the end of the experimental period, rats were lightly anaesthetized with a mixture of Ketamine and Xylazine and euthanized by decapitation. Serum was collected for measurement of Total Antioxidant Capacity (TAC) and kidneys were harvested for gene expression studies using Polymerase Chain Reaction (PCR).
Blood pressure measurement using tail cuff plethysmography
As mentioned above, blood pressure was measured using tail- cuff plethysmography, which is based on recordings of systolic blood pressure according to changes in tail volume (Kent Scientific, Torrington, CT). The method has been validated and recommended by the American Heart Association, particularly for high throughput screening in rodents [12]. All animals were once again allowed to acclimatize to the procedure for 15 minutes prior to the measurement of BP. During this acclimatization, each rat was placed in an appropriately sized cylindrical animal restrainer with the tail exposed at one end. The restrainer together with the rat was placed on an infrared warming platform, measuring 6” x 8”, where the temperature was maintained at 31-32°C to ensure good tail blood circulation. A cuff was then placed around the tail. Twenty recordings were obtained and recorded on the physiography, with a time interval of two minutes between each reading. Three lowest readings were taken and the mean of these was taken as the BP.
1H-NMR analysis of urine samples
Each urine sample was first thawed at room temperature and then 400 μL of the sample was mixed with 200 μL of 0.2 M phosphate buffer (pH 7.40), containing 0.1% TSP. The mixture was vortexed and then centrifuged at 12000 rpm for 5 min. An aliquot of 550 μL from the sample was then transferred into an NMR tube [13]. Urine samples were subjected to NMR spectroscopy at 600 MHz (Bruker 600 AscendTM NMR). The 1H-NMR spectra were recorded at 26oC with a total acquisition time of 2.49 min for 32 scans. The settings for the 1H-NMR spectra were with water suppression pulse sequence 1 dimensional (1D) Nuclear Overhauser Effect Spectroscopy (NOESY)-presat. The pre-saturation was done to suppress the residual water signal. This involves the increment of a NOESY pulse sequence with water irradiation during the relaxation delay and mixing time of 10 minutes. The sweep width, time domain size and Relaxation Delay (RD) were set at 20 ppm, 65 and 2.0 seconds, respectively. To reduce the effect of variation in ionic concentration the bucketing of NMR dataset was done with a bucket size of 0.04 ppm prior to alignment to sodium 3-trimethylsilyl-2,2,3,3-Tetradeuteriopropionate peak. The removed regions were for urea (δ 5.60–5.96) and water (δ 4.70–5.10). The data were converted to ASCII file of the NMR spectra and used in multivariate data analysis using Simca-P software 14 (Umea, Sweden). The data were scaled using Pareto scaling method for all the metabolite signals to have the same importance and reduced noise effects.
Measurement of serum TAC concentration
Total Antioxidant Capacity (TAC) in serum was determined using a commercially available kit (Cayman Chemical, Ann Arbor, MI). Briefly, 10 μl of Trolox standard, 10 μl of Metmyoglobin and 150 μl of Chromogen were added (per well) into the Trolox standard wells. For the sample wells, 10 μl of sample, 10 μl of Metmyoglobin and 150 μl of Chromogen were added. The reaction was initiated by adding 40 μl of Hydrogen Peroxide Working Solution to all the wells. The plate was covered and incubated for 5 minutes at room temperature. Upon removal of the cover, the absorbance was read at 750 nm using a plate reader, Victor X5 (Perkin Elmer, Waltham, MA). The antioxidant concentration was calculated using the equation obtained from the standard curve.
Measurement of GPX, FOXO3a, SOD and catalase gene expression
Gene expressions of GPX, catalase, SOD and FOXO3a were measured using real-time polymerase chain reaction. Extraction and purification of Ribonucleic Acid (RNA) were done using RNeasy Plus Universal Mini Kit (Qiagen) following the guidelines provided in the kit. Briefly, 40 mg of kidney tissue was homogenized using tissue rupture for about 20-40 sec. One hundred μl of g-DNA eliminator solution was added into the homogenate and vortexed for 15 seconds. After extraction, RNA concentration and purity were determined using Nano Drop with the 260/280 ratio of between 1.7 to 2.1. Genomic DNA removal was done using g-DNA Removal Mix (Qiagen) following the provided protocol. Genes examined in this study were normalized with GADPH and TBP genes as housekeeping genes. Total reaction of 20 μl, which included both reverse and forward, was amplified using iQTM real time PCR detection system. Evaluation of the RT- PCR assay for every target gene and reference genes was tested by serially diluting the cDNA from the samples. The RT-PCR efficiency ranging 90-100 % was considered acceptable. The reproducibility or linearity of the method for the samples was tested by calculating the correlation coefficient (R2) and the ones obtained from this experiment fulfilled the desired value of >0.98 as reported in the MIQE guideline.
Statistical analysis
Statistical analyses for blood pressure and TAC were performed using statistical tests contained in SPSS software version 23.0 (IBM Corporation). Differences in blood pressure between the groups were analysed using two-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. Unpaired T-test was used when analysing differences between two groups, with the level of significance set for all analyses at a p<0.05. Data are expressed as mean ± SEM. For the metabolomic study, significant differences among the metabolites were assessed using P values obtained following analysis using ANOVA. The significance among group differences in terms of metabolites were also determined using Metabo-Analyst 3.0, freely available online analysis software.
Effect of FDK extract on mean systolic blood pressure in SHR
Mean systolic blood pressure was significantly lower in FDK- and losartan-treated SHR than that in the non-treated age-matched controls, beginning from week 3 onwards. There were, however, no significant differences in systolic blood pressures between FDK and losartan treated rats over the 4 weeks of treatment. Losartan-treated group was used in this study just to compare its antihypertensive effect with that of the FDK extract. Subsequent analyses of other measured parameters, therefore, just focuses on comparison between the control and FDK treated SHR only (Figure 1).
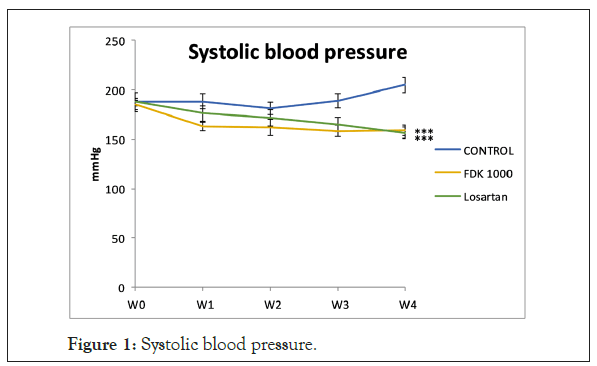
Figure 1: Systolic blood pressure.
Effect of FDK on serum TAC concentration in SHR
Total Antioxidant Capacity (TAC) in the serum of FDK treated SHR was significantly higher (p<0.05) compared to that in the control SHR (Figure 2).
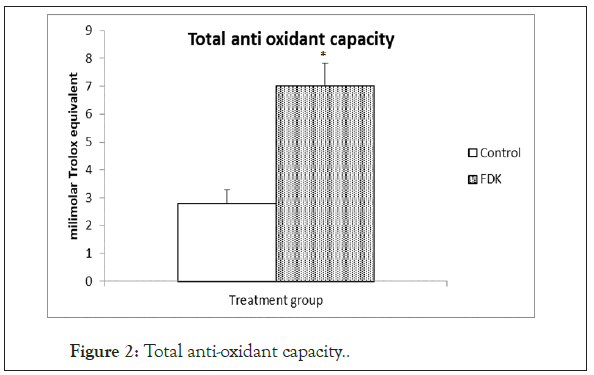
Figure 2: Total anti-oxidant capacity.
Effect of FDK extract of leaves on urine metabolite excretion in SHR
To assess the changes in the metabolomic profile in urine of SHR receiving FDK, 1H-NMR analysis was done. The representative 1H-NMR spectra of control and FDK treated SHR are depicted. Visually, the most intense signals observed within each spectrum are in the carbohydrate region that ranged from 3.00 to 5.00 ppm. There were also small peaks in the regions from 5.00 to 8.00 ppm (aromatic groups) and from 1.00 to 2.00 ppm (aliphatic groups). The intensities of the peaks in the aromatic region were relatively lower than the peaks in the carbohydrate region. Although the NMR spectra of the 2 groups had many similar characteristic peaks, some major signals showed differences between the treated and the non-treated SHR, particularly at peaks 2.5, 2.8 and 3.4 ppm. The characteristic of the 1H-NMR signals was assigned according to Chenomx NMR Suite v. 8.2 (Chenomx Inc., Alberta, Canada), HMDB databases and literature data. This revealed the existence of 47 metabolites, which were present in both groups. Among the endogenous metabolites identified include TCA cycle intermediates such as fumarate, citrate, succinate and α-ketoglutarate, ketone bodies, such as β-hydroxybutyrate and acetoacetate. As the variation between the control and treated group was difficult to observe visually, multivariate data analysis was applied to determine the differences between the groups (Figure 3) (Table 1).
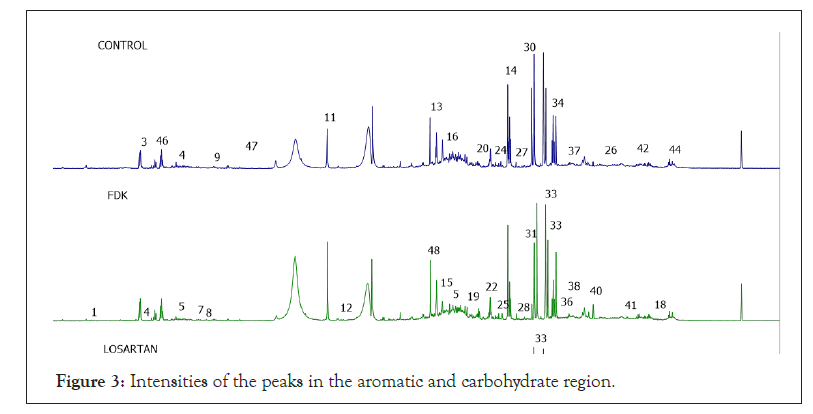
Figure 3: Intensities of the peaks in the aromatic and carbohydrate region.
| Number | Metabolites | H1-NMR characteristics signals | ||
|---|---|---|---|---|
| 1 | Formate | 8.46 (s) | ||
| 2 | Trigonelline | 4.42 (s) | 8.06 (t) | |
| 3 | Hippurate | 3.96 (d) | 7.62 (t) | 7.82 (d) |
| 4 | 3-indoxysulfate | 7.22 (t) | 7.30 (t) | 7.68 (d) |
| 5 | N-phenylacetylglycine | 3.76 (d) | 7.34 (t) | 7.4 (t) |
| 6 | 2-hydroxyphenylacetate | 7.18 (m) | ||
| 7 | Histidine | 7.06 (s) | ||
| 8 | 1-methylhistidine | 6.98 (s) | ||
| 9 | Glutamine | 6.86 (s) | ||
| 10 | Fumarate | 6.50 (s) | ||
| 11 | Allantoin | 5.38 (s) | ||
| 12 | Glucose | 5.22 (d) | ||
| 13 | Creatinine | 4.04 (s) | ||
| 14 | Creatine | 3.02 (s) | ||
| 15 | Betaine | 3.88 (s) | ||
| 16 | Guanidinoacetatae | 3.80 (s) | ||
| 17 | Kynurenine | 3.7 (d) | ||
| 18 | Valine | 0.98 (d) | 3.6 (d) | |
| 19 | Glycine | 3.56 (s) | ||
| 20 | Taurine | 3.42 (t) | ||
| 21 | Acetoacetate | 2.26 (s) | 3.44 (s) | |
| 22 | TMAO | 3.26 (s) | ||
| 23 | Choline | 3.20 (s) | ||
| 24 | Ethanolamine | 3.14 (t) | ||
| 25 | Malonate | 3.10 (s) | ||
| 26 | Lysine | 3.00 (t) | ||
| 27 | Trimethylamine | 2.94 (s) | ||
| 28 | N,N-dimethylglycine | 2.92 (s) | ||
| 29 | Methylguanidine | 2.82 (s) | ||
| 30 | Sarcosine | 2.74 (s) | ||
| 31 | Dimethylamine | 2.70 (s) | ||
| 32 | Methylamine | 2.60 (s) | ||
| 33 | Citrate | 2.54 (d) | 2.70 (d) | |
| 34 | a-ketoglutarate | 2.44 (t) | ||
| 35 | Succinate | 2.40 (s) | ||
| 36 | Pyruvate | 2.36 (s) | ||
| 37 | Acetone | 2.22 (s) | ||
| 38 | Methionine | 2.12 (s) | ||
| 39 | N-acetylglutamate | 2.02 (s) | ||
| 40 | Acetate | 1.92 (s) | ||
| 41 | Alanine | 1.48 (d) | ||
| 42 | Lactate | 1.32 (d) | ||
| 43 | 3-hydroxybutyrate | 1.18 (d) | ||
| 44 | Isoleucine | 0.92 (t) | ||
| 45 | Homocysteine | 3.90 (t) | ||
| 46 | Benzoate | 7.54 (t) | ||
| 47 | Citrulline | 6.40 (s) | ||
Table 1: List of metabolites identified in the OPLS-DA of H1-NMR spectral data of all the treatment groups.
Based on the sets of data from the 1H-NMR spectra, a supervised Orthogonal Projection to Latent Structures-Discriminant Analysis (OPLS-DA) was applied to observe the clustering of the two groups and subsequently to identify the metabolites, which were responsible for the separation between groups. The model is described by PC1 and PC2 with a total variance of 68%. The R2Y and Q2 values are 0.927 and 0.875 and the model was not suffering from over fitting based on the cross validation and misclassification table, which shows 100% correct class. The score plot of the OPLS-DA model of the 2 groups showed clear separation between all the groups. The treated and non- treated SHR (controls) were distinctly separated by the PC2, suggesting that these 2 groups have different sets of metabolites. As demonstrated in the S-plot, metabolite signals, which are positioned far away from the centre of the plot, have a strong impact and play a major role in separating these 2 groups. They also appear as biomarkers of these 2 groups. Among the suggested biomarkers for FDK group are glycine, acetate, succinate, pyruvate and 3-hydroxybutyrate (Figures 4a and 4b).
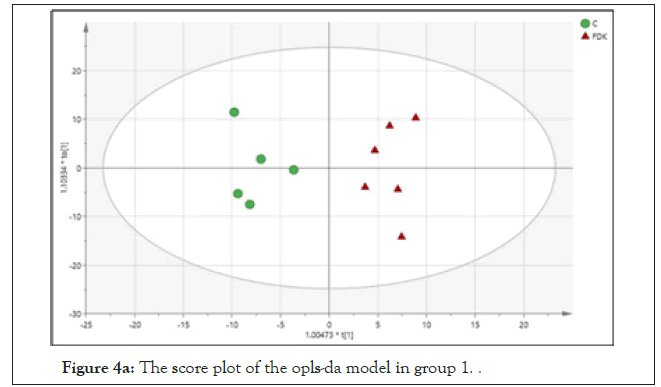
Figure 4a: The score plot of the opls-da model in group 1.
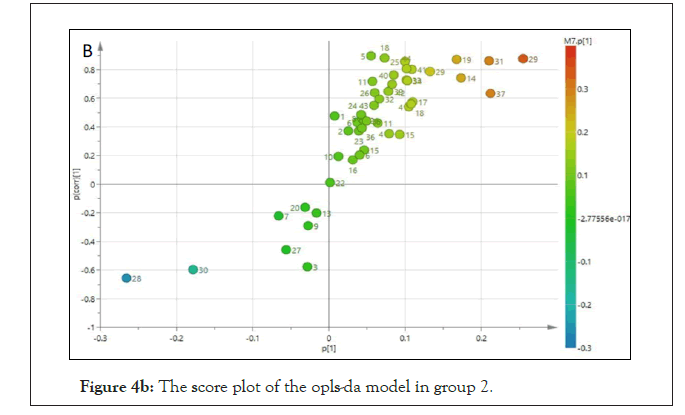
Figure 4b: The score plot of the opls-da model in group 2.
Metabolite significance and relative quantification
Metaboanalyst software was used to determine the metabolites that were statistically significant in differentiating the controls from FDK extract treated groups. Those metabolites with a variable importance for projection (VIP) value of more than 0.5 were considered as significant [14]. Out of the 47 identified metabolites, 35 were statistically significant and might be responsible in discriminating between the control and FDK treated groups. These include metabolites like citrate, succinate, acetate and Dimethylamine (DMA) that had the highest VIP values (VIP>2.0). Other important metabolites were Glycine, Creatinine, Methylamine, Kynurenine, Acetoacetate, N,N-dimethylglycine, Lactate, Isoleucine, Benzoate, Acetone, 3-hydroxybutyrate, Betaine, N-acetylglutamate, Alanine, Hippurate, Homocysteine, Guanidinoacetate, Choline, Valine, pyruvate, Methylguanidine, Lysine, Trigonellinge, N-phenylacetylcysteine, Methionine, Creatine, Malonate, Trimethylamine, 3-indoxysulphate, Glutamine and lastly Allantoin. The red and green colour boxes on the right-hand side of the VIP figure represent high and low level of the respective metabolites. Some of the metabolites, which were significantly higher (p<0.05) in the FDK-treated group include citrate, succinate and acetate, while Dimethylamine (DMA) and methylamine were significantly lower in this group (p<0.05) (Figure 5).
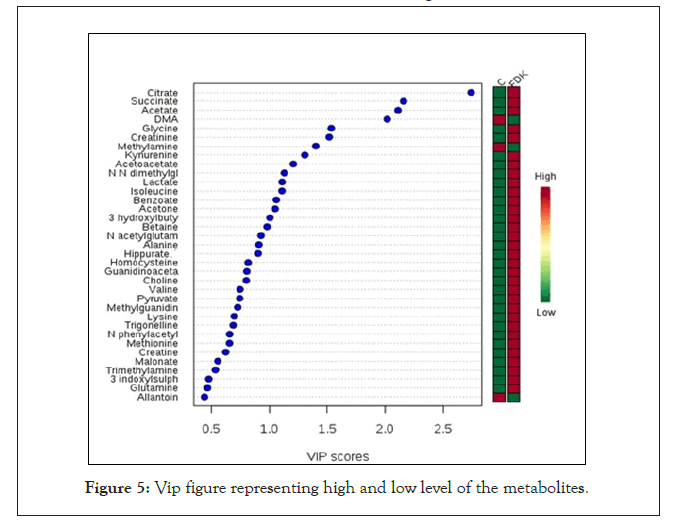
Figure 5: Vip figure representing high and low level of the metabolites.
Correlation of blood pressure and TAC with the urinary metabolites
Correlation among the variables between control and FDK- treated SHR was investigated by Partial Least Squares (PLS) and these variables included blood pressure and TAC. This biplot is a combination of score and loading plots. It shows the differences between the samples and the variables responsible in this separation. Based on the PLS biplot, again it was observed that control and FDK treated groups were distinctly separated in the plot. Higher TAC seems to correlate with the metabolites like Glycine, Citrate, Creatinine, Hippurate, 3-hydroxylbutyrate and Acetate. Dimethylamine and Methylamine correlated well with blood pressure in the control group (Figure 6).
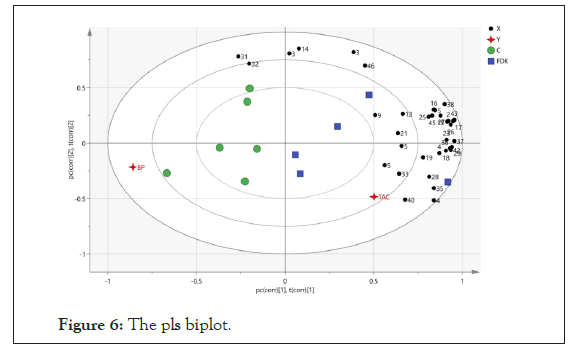
Figure 6: The pls biplot.
The assignments: 1: Formate; 2: Trigonelline; 3: Hippurate; 4: 3-indoxysulphate; 5: N-phenylacetylglycine; 6: 2-hydroxyphenylacetate; 7: Histidine; 8: 1-methylhistidine; 9: Glutamine; 10: Fumarate; 11: Allantoin; 12: Glucose; 13: Creatinine; 14: Creatine; 15: Betaine; 16: Guanidinoacetate; 17: Kynurenine; 18: Valine; 19: Glycine; 20; Taurine; 21: Acetoacetate; 22: Trimethylamine-oxidase; 23: Choline; 24: Ethanolamine; 25: Malonate; 26: Lysine; 27; Trimethylamine; 28: N,N-dimethylglycine; 29: Methylguanidine; 30: Sarcosine; 31: Dimethylamine; 32: Methylamine; 33: Citrate; 34: α-ketoglutarate; 35: Succinate; 36: pyruvate; 37: Acetone; 38: Methionine; 39: N-acetylglutamine; 40: Acetate; 41: Alanine; 42: Lactate; 43: 3-hydroxylbutyrate; 44: Isoleucine; 45: Homocysteine; 46: Benzoate; 47: Citrulline.
Pathway impact analysis
The pathways in which metabolites were found significantly different after treatment with FDK are depicted graphically. The pathways are arranged based on their p-value from the pathway enrichment analysis and pathway impact scoring. The circle signifies the metabolism, and ranked based on the colour gradient (yellow: greater p-values, red: lower p-values). Administration of FDK extract to SHR changed the urinary metabolite profile of the rats and these changes involved metabolic pathways of amino acids (Valine, Leucine and Isoleucine biosynthesis, Glycine, Serine and Threonine metabolism, Alanine, Aspartate and Glutamate metabolism), for synthesis and degradation of ketone bodies, Butanoate metabolism and pyruvate metabolism. Collectively, the involvement of these pathways indicates potent antioxidant effects of FDK extract. Since Fork head box O (FOXO) 3a, SOD2, catalase and glutathione peroxidase are the key antioxidant defenses, mRNA expression for these parameters in the kidney tissue was determined (Figure 7).
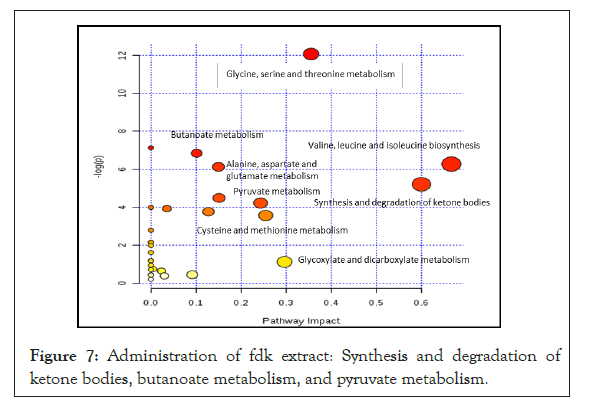
Figure 7: Administration of fdk extract: Synthesis and degradation of ketone bodies, butanoate metabolism, and pyruvate metabolism.
Effect of FDK extract on the mRNA expression of GPx, FOXO3a, MnSOD and catalase in the kidney of SHR
No difference was seen in the renal mRNA expression of GPx between control and FDK treated groups, although a slightly higher mean value in the FDK treated group was observed. However, renal mRNA expressions for MnSOD (p<0.05), catalase (p<0.05) and FOXO3a (p<0.05) genes were significantly greater in FDK treated SHR compared with those in the control SHR (Figure 8).
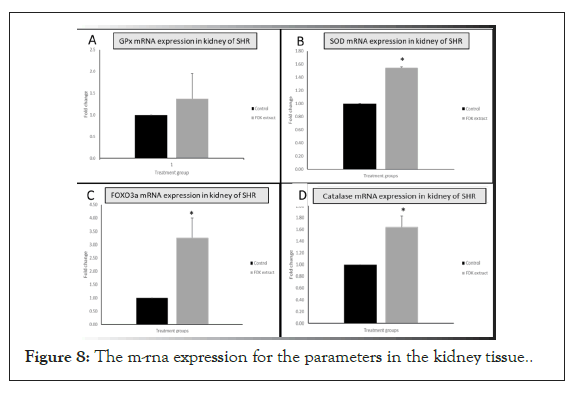
Figure 8: The m-rna expression for the parameters in the kidney tissue.
We had earlier reported that the blood pressure lowering effect of FDK involves changes in the renin-angiotensin-aldosterone system and improvement of endothelial function [11]. The blood pressure lowering effect of 1000 mg.kg-1 day-1 of FDK was once again comparable to that of 10 mg.kg-1 of losartan per day. However, further analysis of serum, tissue and data from urine metabolite analysis of rats treated with FDK, in the present study, seems to suggest that the anti-hypertensive effect of FDK may also involve the antioxidant system, and perhaps other unidentified mechanisms too. TAC was higher in FDK treated rats. In addition, mRNA expressions of some of the antioxidant enzymes were also higher following FDK treatment. There were also significant differences in urine metabolite profiles between control and FDK treated rats. Some of these metabolites include amino acids, ketone bodies and those involved in energy metabolism. A number of these metabolites have been shown to affect blood pressure. Collectively, the results seem to suggest that the blood pressure lowering effect of FDK might involve multiple mechanisms or mode of actions.
Effect of FDK extract on the Total Antioxidant Capacity (TAC) and expressions of antioxidant enzymes
TAC was significantly higher in FDK treated rats. TAC is a measure of radical scavenging capacity of a sample in vitro. Although its extrapolation to the antioxidant defense system in vivo is still a matter of some debate, it nevertheless is often used as a measure of antioxidant status in vivo together with the measurement of antioxidant enzyme activities. The most likely reason for the raised serum TAC in FDK treated rats could be the greater presence of polyphenols and flavonoids in the serum following the ingestion of the FDK extract. Polyphenols and flavonoids are well known for their antioxidant properties [15,16]. The levels of these phytochemicals were not measured in this study but the leaves of FDK are reportedly rich in polyphenols and flavonoids. Apart from directly scavenging or quenching radicals like ROS/NOS, flavonoids and polyphenols also inhibit NADPH oxidase, suppress ROS production, and inhibit eNOS uncoupling [17-19]. In addition, polyphenols and flavonoids from various sources have been shown to increase the expressions of SOD2 and catalase [20,21]. SOD2 and catalase mRNA expressions were higher in FDK treated rats. Both SOD and catalase are ROS detoxifying enzymes. Increased expression of these endogenous antioxidant enzymes and their activities will increase the capability of the antioxidant defence system in vivo. Antioxidant properties of FD extract have been reported before [22,23].
The precise mechanism responsible for the increased mRNA expression of these antioxidant enzymes following the ingestion of FDK is unclear but might involve FOXO3a. FOXO3a mRNA expression was significantly higher in FDK treated SHR compared to that in the non-treated SHR. FOXO3a protein is a member of the FOXO subclass of transcription factors. It is a regulator of genes involved in central biological processes including cell turnover. ROS, incidentally, also promote phosphorylation of FOXO3a. The latter then translocate to the nucleus and increases the transcription of SOD2, catalase and Glutathione peroxidase [24]. During conditions of oxidative stress, FOXO3a has been shown to increase the transcription of SOD2 [25,26]. Flavonoids have been found to induce the transcription of FOXO and to also inhibit PI3K/Akt pathway [27,28]. FOXO3a activity has been shown to be mediated by Phosphatidyl-inositol 3-kinase (PI3K)/Akt (also known as Protein kinase B) signalling pathway [29]. Inhibition of PI3K/Akt pathway enhances the binding of FOXO3a to the SOD2 promoter [30].
In view of the raised TAC, increased expression of some of the antioxidant enzymes and information from the literature, it seems that FDK reduces oxidative stress, which helps reduce blood pressure. Oxidative stress has been implicated in hypertension, and numerous investigations have pointed to increased oxidative stress in hypertension both in humans and SHR [31-35]. Antioxidant therapy has been suggested as a potential treatment of hypertension, although reports from studies on the impact of antioxidant therapy on blood pressure remain equivocal at the moment [36].
In order to identify further possible mechanisms underlying the anti-hypertensive effect of FDK extract, urine metabolite profile was determined using 1H-NMR spectroscopy. A total of 47 metabolites were identified in the urine of both treated and non-treated SHR based on HMDB and the literature. Differences in the urine metabolites profile of FDK treated SHR included differences in some amino acids, ketone bodies and energy metabolism. Based on the Partial Least Square (PLS) analysis, the metabolites with higher VIP values were found to be well correlated with the 2 important parameters examined in this study, which are blood pressure and TAC. Based on the pathway impact analysis, among the highest pathways involved in the changes in metabolites in the FDK treated SHR are ketone bodies synthesis, branched chain amino acids and glycine, serine and threonine metabolism. The details of these pathways are discussed in the following sections.
Synthesis and degradation of ketone bodies: hydroxybutyrate, acetoacetate and acetone
Administration of leave extract of FDK to SHR resulted in increased excretion of 3-hydroxybutyrate (also called β-hydroxybutyrate), acetoacetate and acetone in the urine. Ketone bodies are central in physiological homeostasis. They are synthesized in the mitochondria of liver cells. There is also growing evidence supporting a significant antioxidant activity of ketone bodies [37-39]. It is possible FDK extract could have decreased the oxidation of 3-hydroxybutyrate, thus increasing its level in the serum and its excretion in the urine. Cardio-protective effect of β-hydroxybutyrate from ischemia/reperfusion injury in mice has been reported [40]. Lower level of β-hydroxybutyrate has been observed in the rat model of acute myocardial infarction, suggesting its probable protective role in the cardiovascular system [41]. β-hydroxybutyrate has also been shown to possess histone deacetylase (HDAC) inhibitor activity. β-hydroxybutyrate, has also been reported to upregulate gene expressions of FOXO3a, Mt2, Lcn2, Lemd3 and Hbp1 [42]. It is, therefore, possible that the increased expression of FOXO3a, SOD2 and catalase following treatment with FDK could be due to the higher levels of β-hydroxybutyrate, which may have also contributed to the anti-oxidant action of FDK.
Glycine, serine and threonine metabolism
Glycine content in the urine was significantly greater in FDK treated rats compared to that in the non-treated controls. Glycine is a non-essential amino acid. It is the smallest amino acid and plays a crucial role in the synthesis of several biologically important compounds. Elevated glycine levels in the urine of FDK treated SHR may have been derived from either enhanced metabolic breakdown of Serine, Threonine and Choline or from the FDK extract or both. FDK is a source of not only glycine but also Serine and Threonine, as plant proteins, including those in FDK, are rich in Glycine, Serine and Threonine [43].
Glycine is a component of GSH, an important member of the oxidative defense system in the body. Glycine is required for GSH synthesis [44]. Serine and threonine, the precursors of glycine, also contribute to GSH synthesis. Glycine prevents ROS formation by inhibiting the activation of NFKB and restores plasma nitrites and nitrates levels to normal [45]. Serine and threonine are also essential for glutathione synthesis via conversion to glycine. In fact, serine was shown to reduce oxidative stress in mice and primary hepatocytes by restoring the depleted GSH levels after exposure to Diquat [46]. In addition to its role in GSH synthesis via its metabolite glycine, supplementation with threonine in diet was also shown to increase SOD activity and total antioxidant capacity and lower MDA levels in hens [47]. Clearly, all three amino acids, glycine, serine and threonine play an important role in antioxidant defenses. The significantly greater total antioxidant capacity observed in FDK treated SHR may have resulted from its ability to increase glycine, serine and threonine levels. It is also interesting to note that higher urinary excretion of glycine in FDK treated SHR also supports its benefits in the complications associated with hypertension, as studies have shown that glycine was not only found to be inversely associated with the risk of cardiovascular diseases, but it also confers some protection against cardio-metabolic diseases [48,49]. Overall, it can be speculated that relatively higher urinary levels of glycine in FDK treated rats may be derived from the extract. Glycine in fact may have an important role in promoting the antihypertensive effects of FDK extract via enhancement of antioxidant defenses.
Branched chain amino acids
Administration of FDK extract to SHR also led to higher urinary excretion of Branched-Chain Amino Acids (BCAAs) particularly Valine and Isoleucine. Valine, Leucine and Isoleucine are essential amino acids in animals. They are significant sources for the biosynthesis of sterol, ketone bodies and glucose. It is highly likely that the higher levels of BCAAs in the urine of FDK treated SHR came from the extract itself. There are conflicting findings on the association of BCAAs and cardiovascular diseases in human and animal studies [50,51]. Lower concentration of Valine has been reported in plasma of adult SHR when compared to that in age-matched normotensive controls [52]. This might suggest a role for Valine in the regulation of blood pressure. Other studies have shown a positive relationship between the levels of BCAAs and hypertension [53]. Essentially, it is crucial that BCAA homeostasis is maintained, as dysregulation of BCAA metabolism may lead to an increase in the levels of free BCAAs or its catabolic products that can be cytotoxic. Several known diseases such as maple syrup urine disease and Methylmalonic acidemia are due to a defect in the BCAA catabolic pathways. These diseases are associated with increased levels of BCAA in the body as well as disturbances in central nervous and cardiovascular systems. Dysregulation of BCAA catabolism has also been shown to disrupt the metabolism of glucose and sensitize the heart to ischemia-reperfusion injury [54]. In the current study, a higher urinary concentration of citrate and succinate was observed in FDK treated SHR compared with that in non-treated SHR. It is likely that the circulating BCAAs are being catabolized, and their catalytic end products including Acetyl-CoA and Succinyl-CoA, are subsequently converted to citrate and succinate, respectively and appear in the urine. Hence the higher urinary excretion of BCAAs, and also their increased catabolism, reflects BCAA homeostasis secondary to their increased intake with the FDK extract. It is also, however, notable that BCAA supplementation to male Wistar rats with advanced liver cirrhosis causes increased expression of SOD1 and SOD2 and ROS defence system in the liver [55]. BCAA also possess ROS and NO scavenging properties. In a study in vitro, GSH levels were found to be positively correlated with the levels of BCAAs [56]. It is, therefore, possible that FDK extract by helping maintain BCAA homeostasis exerts ROS and NO scavenging effects thereby protecting the endothelium from oxidative damage and improving endothelial function.
Pyruvate metabolism
Higher levels of pyruvate in the urine were also evident in FDK treated rats when compared with that in non-treated rats. Pyruvate is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. It is widely known to play a critical role in numerous human and eukaryotic metabolic reactions. The reason for the relatively higher levels of pyruvate in FDK treated SHR compared to those in the non-treated SHR is unclear. The results of the current study, nevertheless, correspond to findings of another study in the literature that showed significant perturbation in metabolites like alanine, arginine, methionine, pyruvate, adenine and uracil on NMR-based analysis of filtered serum samples of patients with essential hypertension and healthy individuals [57]. Pyruvate, alanine, methionine and uracil were found to be lower in the serum of patients with essential hypertension suggesting the association of these metabolites with changes in blood pressure. Other studies have also reported lower pyruvate level and higher lactate/ pyruvate ratio in both hypertensive human subjects and hypertensive animal models [58,59].
It is important to note that under conditions of oxidative stress, the activity of pyruvate kinase, the key enzyme of pyruvate synthesis, is inhibited across diverse organisms ranging from Escherichia coli to humans, leading to reduced production of pyruvate [60-63]. It is likely that in non-treated SHR, there was inhibition of pyruvate kinase due to greater oxidative stress resulting in relatively lower levels of pyruvate. The higher antioxidant activity in FDK treated rats may have reduced the oxidative stress, which in turn increased pyruvate kinase activity and pyruvate levels leading to its increased urinary levels. It is also important to note that pyruvate by itself has been shown to possess potent antioxidant properties due to its α-ketocarboxylate structure, which enables it to directly neutralize peroxides and Peroxynitrite.
Evidence showing an association between reduced pyruvate levels and increased oxidative stress in SHR also comes from several studies reporting an association between ATP production and oxidative stress [64,65]. In fact, examination of the brain stem, a central regulator of cardiovascular homeostasis and systemic blood pressure, in SHR revealed that reduced ATP synthesis is associated with occurrence of mitochondrial complex I dysfunction, high ROS production, as well as impaired mitochondrial respiration [66]. These studies clearly indicate the association of reduced pyruvate level with oxidative stress. In FDK treated rats, restoration of redox status was, therefore, associated with increased pyruvate levels. Furthermore, it appears that increased pyruvate level stimulates glycolysis by increasing the availability of Acetyl-CoA, which enters the Krebs cycle to restore ATP production and reduce oxidative stress. Other than pyruvate and Alanine, Urinary citrate and Succinate levels were also greater in FDK treated rats [67]. Citrate and Succinate are the intermediates of TCA cycle and their increased level in the urine of FDK extract treated SHR suggests a robust TCA cycle activity, which is likely to result from increased pyruvate levels.
Taken together, it can be concluded that administration of 1000 mg.kg-1 body weight of standardized aqueous ethanolic extract of Ficus deltoidea kunstleri to SHR reduces blood pressure. Its blood pressure lowering effect also involves increases in antioxidant activity as shown by a higher total antioxidant capacity and changes in urinary metabolite excretion, particularly those that are related to increasing the anti-oxidant status. These changes involve the metabolism of amino acids, ketone bodies and energy metabolism. Further studies are required to assess possible involvement of other metabolic pathways in the blood pressure lowering effect of the FDK leaf extract.
This study was supported by the Ministry of Agriculture (MOA) (100-RMI/MOA/16/62).
The authors have no conflict of interest to disclose.
Citation: Singh HJ, Azis NA, Kamal MSA, Mediani A, Agarwal R, Ismail NH, et al. (2021) Identification of Antihypertensive Mechanisms of Ficus deltoidea kunstleri in Spontaneously Hypertensive Rats Using Metabolomics. J Plant Biochem Physiol. 9:264.
Received: 21-Jun-2021 Accepted: 05-Jul-2021 Published: 12-Jul-2021 , DOI: 10.35248/2329-9029.21.9.261
Copyright: © 2021 Singh HJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited