Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2019)Volume 11, Issue 2
Aim: Based on this hypothesis a new biological combination termed V20E immune peptides comprising HIV viral antigen and a non-specific HIV antibodies has been formulated in oral and injectable form for inhibiting or preventing the HIV infection.
Introduction: A new assumption assumes that CD4+ T-cell can induce the production of a pathogenic broadly neutralizing anti-HIV antibodies to mask a proportion of corresponding HIV antigens in immune-complex form (Ag/ nAbs) antigen/neutralizing antibody for a long time preventing its attacks by CD8+ cytotoxic T-cells and anti-viral drugs.
Materials and Methods: A pilot study was carried out on a total of twenty-five patients (21 males, 4 females; aged 28-38 years) all of them were positive for HIV antibodies and divided them into three groups I, II and III, each group included seven patients. Only four HIV-positive plasma patients as control (IV group) were advised to take Antiretroviral drugs (ARVs) and enrolled on to a similar protocol. The groups were classified according to their CD4+, CD8+T-cells count, quantitation of HIV RNA and showed the same clinical symptoms of HIV/AIDS. All the patients wrote consent of acceptance to take the V20E immune peptides combination therapy in the form of S/C Injection or oral capsules for 12 weeks.
Results: Serum samples were collected 3 times for Quantitative measuring of (HIV RNA), circulating immunecomplex (CIC) IC1, IC2, and IC3, CD4+ and CD8+ T-cells count. At the end of this therapy, all of the patient's viral loads had reached under the detectable limits (less than 50 copies/ml); also there were significant Increases of their CD4+ T-cells count.
Conclusion: These results have important implications for the development of real therapeutic and prophylactic vaccine and also raise the great possibility for developing a serological screening method for monitoring HIV infection and the successful treatment.
Vaccine; CD8+ Cytotoxic T Lymphocyte; CD4+ Helper Cells; Neutralization Antibodies; Gp120 Antigen; P24 Antigen; Anti-P24 Antibodies; Anti-GP120 Antibodies; Immune-complex.
AIDS: Acquired Immune Deficiency Syndrome; ARVs: Anti-retroviral Drugs; HIV: Human Immune Deficiency Virus; ART: Antigen-Antibody Complex Ag/Abs; AR: Antiretroviral therapy; CIC: Circulating Immune Complex; F1: gp120 antigens and Abs of p24; F2: p24 antigen and Abs of gp120 (F2), F3: Reverse Transcriptase Enzyme of HIV-1 and Abs of p24.
HIV continues to be a major global public health issue. In 2017 an estimated 36.9 million people were living with HIV (including 1.8 million children) with a global HIV prevalence of 0.8% among adults [1]. Envelope glycoprotein gp120 is one of HIV antigens [2]. gp120 is essential for virus entry into cells as it plays a vital role in attachment to specific cell surface receptors. The HIV envelope glycoprotein, gp120, has been shown to inhibit T-cell function and induce the expression of proinflammatory and immuno-regulatory cytokines in vitro. p24 antigen Gag protein from HIV-1 is a polyprotein of 55 kDa which, during viral maturation, is cleaved to release matrix protein p17, core protein P24 and nucleocapsid protein p24 antigen is the core protein of the human immunodeficiency virus (HIV) [3,4]. p24 plays an important role in viral core assembly and maturation, the presences of P24 antigen in the blood is a marker of uncontrolled HIV replication, P24 antigenemia is encountered in the acute retroviral syndrome before host immune response and in advanced AIDS when the immune system has been destroyed. When p24 antigens detected in the blood, the HIV viral load is high and the person is highly infectious.
Reverse transcriptase is an enzyme encoded from the genetic material of retroviruses that catalysis the transcription of retrovirus RNA (ribonucleic acid) into DNA (deoxyribonucleic acid), reverse transcriptase is central to the infectious nature of retroviruses [5,6].
All studies have been providing us with the evidence that neutralizing antibodies are potent enough to prevent HIV infection, and strongly suggest that neutralizing-antibody-based vaccines could provide effective protection against HIV-1, despite the potent action of CTLs [7].
Immune-complex rises when the body's immune system generates antibodies against antigenic determinants of host or foreign substances that recognize and bind to the antigen molecules an immune-complex is formed which comprises this neutralizing antibody/Antigen complex. Normally, insoluble immune complexes that are formed are cleared by the phagocytic cells of the immune system, but when an excess of antigen–antibody are present, the immune complexes are often deposited in tissues [8,9], where they can elicit complement activation, localized inflammation resulting in the generation of tissue lesions in a variety of autoimmune diseases, exacerbating disease pathology [8,9].
In study of how the immune system copes with virus infection is a key to understand virus replication strategies and their overall structure along with why certain populations of cells are targeted for infection, and the selection of virus mutants with growth advantage. Consequently, deciphering how the immune system functions assists in understanding how viruses evolve to survive and propagate in hosts. CD4+ T-cells impact antiviral immunity at multiple stages of the immune response. CD4+ T cells influence antiviral cellular and humoral immunity [10]. CD4+ Tcells enhance early expansion of virus-specific primary CTL, their subsequent differentiation into memory cells, guide their localization to sites of infection, and drive secondary expansion of memory CD8+ T-cells during re-infection [11].
Do neutralizing antibodies influence HIV replication?
Understanding HIV mode of action and its strategy will reduce more researches efforts, and can open the gate for developing an effective vaccine or therapy that could help to reduce the severity of the disease and prevent the spreading of infection. Our new approach states that the evolution of the virus originated from the core of the cell, furthermore, it uses a body self-cell which is CD4+ T-cell to develop a new behavior completely different from its primitive behavior that naturally should be refused and attached by the controller CD8+ T-cell to keep the innate entity, that new behavior and mutation within the cell principle enabled CD4+ T-cell to produce Agent in form of RNA codes used by the cell to transport its DNA sequence as a conqueror agent and colonizes itself within the CD8+ T killer cell to change its program, forcing the cell to execute its own orders. The mutation strategy for CD4+ T-cell was the main reason behind the capability of the virus to stay within the body for a long period of time without being intact, all of that is related to the indispensable part of CD4+ T-cell in keeping the virus alive and persist in producing it. But here is the most important question, if these cells are the agents responsible for the virus production and maintaining it safe, what is the reason behind the production? The theory of the virus initiation is based on that permanent exposure of the CD4+ T-cell to many new and abnormal effectors, these effectors trigger the CD4+ T-cell to modulate its natural physiological behavior to keep it immunized from the attack of the killer cells by using codes and DNA sequences to find a way to change the killer activity of CD8+ T-cell but there is a point the CD4+ T-cell did generate an agent and fortified it with a protective codes to regenerate a new entity able to enter the killer cells, executing the orders wanted by this cell, by this process it transports itself within the killer CD8+ T-cell using that viral agent assuring its complete captivation of these CD8+ T-killer cells, but there is a point, The CD4+ T-cell that desired the changes Insured the success and fulfillment of its own plan but it has no idea about the resulting consequences, it only focuses on the relief from the problem which is the attacking action of CD8+ T-cell and giving a side for the importance to know the result of that abnormal behavior on the whole body mechanisms, resulting in a complete imbalance of the body immune system. We can imagine the state as a small rat biting an electric wire which serves a huge building, that rat has no idea about the amount of loss it can cause. Let us think what if our CD4+ T-cell fail in finding a way to protect itself, a cellular collusion will be the body status, our body cells will start to fight each other’s during the absence of the natural body solutions to protect itself. That’s why these CD4+ T-cells tried on creating that agent to protect it from confronting the killer cell. CD4+ T-cell are forced to approach this attitude, as in case of the cancer cell which is turned up from a normal cell into a cancer cell due to the continual exposure for exterior effectors, making it unable to find a solution, at the same time if it undergoes any change definitely will result in an attack by the immunity to destroy it. This approach may help us to understand the initiation of the viruses and reconsider a new way in thinking! This new Postulation may lead us to think logically and make us say the virus which we try hard to kill by different strategies, it can be one of our cellular choices and all the different types of the therapeutically approaches would cause a harmful effect on our immune cells. This may justify the failure of all the previous attempts to find a real cure for this virus. Pertaining to previously discussed points, a new mode of action for HIV was arising that incriminate the neutralizing antibodies and the CD4+ T-cells as the main cause for the persistence of the HIV infection. During HIV infection the broadly neutralizing antibody that are generated it existed in two forms, the bounded form where it effectively mask a proportion of corresponding HIV antigens prevents its elimination, controlling its activities and a free form which is a freely neutralizing Abs. The scenario of infection transmission start when the CD4+ T-helper cells send an immunological signal for the B-cells to produce a broadly specific bounded-nAbs for every structural proteins parts of HIV, these antibodies has the same nature and structure but differ in their physiological behavior and their nature may reflect the immunity of the CD4+ T-helper cells. If the B-cells received a negative signal from infected CD4+ T-helper cell, it translated this negative signal to nAbs productions, this nAbs has the ability to induce the infected CD4+ T-cell to mutates to become a modulated version of CD8+ T-cell and these mutated CD4+ T-cell is now considered to be CD8+ T-cell but with the absence of the same physiological behavior, functions, and totally dissimilar from the original CD4+ T-cell [12]. The loss of CD4+ T-cell cause a complete cellular discrepancy and a duplication in the cell signals, as that the newly formed CD8+ Tcell may permit an activator signals while the original CD8+ Tcell inhibits these signals. This imbalance leaves the immune system in a state of confusion with two contradictory responses that directly cause deregulation and an ultimate failure of the host cellular immune networks. On the contrary, immunocompetent CD4+ T-cell activate the production of the positive nAbs from B-cells, it directly stimulate the CD8+ CTL to clear the HIV.
How close are we to a 'Real Cure' for HIV?
V20E immune peptides mode of action: Depends on question doesn’t answered yet, why the killer cell cannot get rid of the HIV and expel it and why most of HIV-1- infected individuals which mount a more vigorous bnAb response not able to stop the viral infection?, our new hypothesis believed that the answer is stored inside the CD4+ T-cell cell and its relation with the virus, so to find a way for real cure, we have to force the CD4+ Tcell to send a positive bnAbs, this will make the viral antigens coverless without protection.
This study was specifically designed to study the reasons that make the HIV has all these abilities to continue and stay for long years in spite of all immune cells weapons and the huge broadly neutralizing antibody, how it adapt to the concealment and mutate. In this article we reveals a new insight for the role of antibodies which is produced by the B-cells as a main cause in persisting the viral infection by coating the viral antigens particles for a long time as Ag/Abs in a complex form protecting it against attacks of the CD8+ cytotoxic T lymphocytes (CTL), CD4+ T-helper cells and the anti-viral drugs, also this study focusing on using a novel biological peptides combination and its positive results in elimination the viral particles and can restore the CD4+ T-cells to normal value, also the novel results can raising the possibilities of using these complexes structure in formulating a vaccine for curing of HIV.
This study was applied in R and D center for HIV researches between October 2015 and March 2016. Subjects with any chronic diseases were excluded (Diabetes, renal & liver affection, hypertension, cancer). This study was carried out on a total of twenty-five patients (21 males, 4 females; aged 20-38 years) (Table 1). All of them were positive for HIV antibodies all of the subjects participated in this trail after giving informed consent about the trail and the novel treatment. All infected participants were divided into three Groups I, II and III every group contains seven patients, the control group were already registered under surveillance by HIV/AIDS Control Department at the Egyptian Ministry of Health (MOH) to take the Antiretroviral drugs (ARVs) enrolled to the same protocol as a control group IV, the groups were classified according to their CD4+, CD8+ T-cells and quantitation of HIV RNA.
| Lab | Measures | Group-I | Group-II | Group-III | p-value |
|---|---|---|---|---|---|
| Age (years) | Mean ± SD | 27.6 ± 5.5 | 28.9 ± 5.6 | 28.3 ± 6.0 | ^0.915 |
| Range | 20.0–38.0 | 22.0–37.0 | 20.0–36.0 | ||
| Sex (n, %) | Male | 6 (85.7%) | 5 (71.4%) | 6 (85.7%) | #1.000 |
| Female | 1 (14.3%) | 2 (28.6%) | (14.3%) |
Table 1: Age and sex among the studied cases.
V20E immune peptides
V20E immune peptides protein combination compose of a unique and highly complex mixture comprise antigens and their nonspecific neutralizing antibodies to be injected (S/C, I/M or I/D) or orally as capsules form eliciting our immune response to secrete antibodies against it. The present invention therapy introduces three combinations, the first one comprise [gp120 antigens and Abs of p24] as (F1). The second combinations comprise [p24 antigen and Abs of gp120] as (F2) and the last one has reverse transcriptase enzyme of HIV-1 and Abs of p24 as (F3). The present combinations were prepared as capsules, vial form and subjected to the standardization of the manufacturing instructors’ as pharmaceutical form [13].
Immune-complex in vitro formulation
HIV antigens (gp120, p24 and RT) of different concentrations (0.05, 1, 1.5 ug/ml) and its antibodies (anti gp120 antibody anti p24, and anti RT) of concentrations ranges from (0.05 to 5 ng/ml) of (SIGMA-ALDRICH) were obtained to make a vitro serial concentration of agglutinate immune-complex IC1, IC2, and IC3 (antigen/antibody). An equal concentration of antigens and its specific antibodies were mixed to allow for agglutination and precipitation, to obtain three specific different complexes structure, IC1 (gp120 antigen/ anti gp120), IC2 (p24 Ag/anti p24) and IC3 (RT antigen/anti RT antibody) with these serial concentrations; 0.05, 0.5,5,10,15,20 ng/ml (Every concentration of antigen is incubated to its similar concentration of Abs, a polyethylene glycol was added to maintain the activity of the structural protein, then the mix were centrifuged at 2500 g for 10 minutes, the supernatant are poured and the obtained sediments were added to 3 ml of Freund's complete adjuvant to immunize the rabbits groups with these immune-complexes to induce the production of IgG-anti complex antibody.
Immunized lab animals with the prepared complexes peptide (IC1, IC2, and IC3)
A 24 male New Zealand albino rabbits weighing 8-10 lb were classified into three groups (A), (B) and (C), every group contains 8 rabbits and every group of rabbits injected with specific complex. Were Group (A) injected with Complex IC1, group (B) injected with complex IC2 and groups (C) with Complex IC3? All the rabbits in every group were injected with 0.20 ml of its specific complex intramuscular for 45 days, after six weeks blood samples were collected from the rabbits and 30 ml of immune serum (150 mg) was obtained from every rabbit to isolate the IgG anti-complex antibody, were all rabbits of group (A) has IgG anti-IC1, group (B) IgG anti-IC2 and the group (C) has IgG anti-IC3 antibodies [14,15].
Ammonium sulfate 40% saturated solution was used to precipitate the antibody, we centrifuging the precipitate at 15,000 rev/min for 5 minutes in a Beckman 152 Microfuge, the supernatant was removed and the precipitate was washed twice with half-saturated (NH4)2SO4, then we carefully remove and discard the supernatant, we resuspend the precipitate with PBS, another centrifugation was done to remove any remaining debris to obtain the anti-complex antibody from sera of all rabbit in each group, we determined the IgG concentration (ng/ml) by estimating the Absorbance reading at 280 nm.
Detection of (IC1), (IC2) and (IC3) in serum samples of infected patients in all examined groups
A 96-well plate coated with IgG anti [IC1, IC2 and IC3] antibody that obtained from the rabbits blood to capture the different complexes in serum samples of infected patients with HIV.
Description: The ELISA Assay Kit was designed to measure the quantities of IC1, IC2, and IC3 in serum samples of the infected groups. We designed the supplied components in every kit as follow:
• ELISA plates Kit Components for detection of IC1, included a-Component A coated plate with 50 μl Anti IC1, Component B is a Complex C1 Standard calibrators (0.05, 0.5, 5, 10, 15, 20 ng/ml).
• ELISA plates Kit Components for detection of IC2, included a-Component A coated plate with 50 μl Anti IC2, Component B is a C2 Standard calibrators (0.05, 0.5, 5, 10, 15, 20 ng/ml).
• ELISA plates Kit Components for detection of IC3, included a-Component A coated plate with 50 μl Anti C4, Component B is a IC3 Standard calibrators (0.05, 0.5, 5, 10, 15, 20 ng/m), and the other kits components included Component C: HRP-Conjugated Anti-Rabbit IgG 25 μl, Component D: TMB Solution A (3, 3', 5, 5'- tetramethylbenzidine) 15 ml, Component E: TMB Solution B (H2O2) 15 ml, Component F: TMB Stop Solution 30 ml.
Exploring the presence of (IC1, IC2, and IC3) in serum samples of HIV infected patients.
We run the three ELISA assay to detect each specific complex in serum sample for all patients in each group (I, II and III). We diluted the complex [IC1, IC2 and IC3] Standard (Component B) and every examined serum sample with PBS to ten concentrations, then 100 μl of diluted Standard, samples were added to the specific well (component A) of the three designed kits. Incubated at room temperature for at least 1 hour, Dilute 10 μl of Anti Rabbit IgG (Component C) with 10.5 ml of Diluted Solution then we added 100 μl of HRP-Conjugate to each well. Aspirated and washed 4 times, and then incubated at room temperature for 30 minutes, 100 μl TMB Peroxidase substrate Solution (Component E) was added to the wells, and then it was incubated at room temperature for another one hour, then and each well was estimated at absorbance of 450 nm using an ELISA reader (Bioteck, USA). The mean OD readings of complex C1, C2, and C3 were significantly higher than (p<0.05) which is considered positive among all infected HCV patients.
Detection of free anti-HIV in serum samples of the infected patients
A device comprising three plates connecting to each other was used, were every plate coated with 50 μg/ml of specific antigen (gp120, P24 and RT antigens) to capture its specific circulating antibodies in the HIV positive sera. To prepare the immune reactive plate, 50 μg/ml of each specific antigen was added to adsorb in to the micro plates for 48 hrs at 4°C. Wells were washed with PBST, a 100 μl of each tested serum samples of HIV was added for every triple wells for all groups (I, II and III); we incubated the plates at 37°C for 1:30 hrs. Then we washed them, after that we incubated them with 100 ml rabbit anti-human IgG conjugated (HRP) at 37°C for 30 min. The immune complexes were detected by adding 100 ml of TMB substrate solution, the wells were washed, and then the plates were measured by using an ELISA reader (Bioteck, USA) at 450 nm absorbance. To distinguish between positive and negative HIV sera the cutoff value was calculated as the mean value of the OD of HIV 150 negative samples plus 2 Standard Deviations (SD) equal to 0.05 ng/ml.
Counting of CD4+ T-cells and CD8+ T-cells
With using simplified thermo resistant assay (STRA), fifty microliters of whole blood (along with K3EDTA) is added to the test tube that contains the pre dispensed, stabilized monoclonal antibody. After 15 min of incubation, the blood samples are diluted 1:10 using a phosphate buffer and analyzed on a flow cytometer. Sample analysis is performed by a comparison of the specific fluorescence signal attributable to the presence of CD4+ or CD8+ antigen at the cell surface with the side scatter signal [16,17].
Quantitation of HIV-1 RNA
The QUANTIPLEX HIV-1 RNA assay, version 3.0 (bDNA 3.0 assay), was performed according to the manufacturer’s instructions provided with the assay kit. The limit of detection of the assay indicated by the manufacturer was (50 copies/ml). All tested group were measured the quantitation of HIV RNA before starting the intervention therapy, 12 weeks after the last dose of the therapy and 24 weeks from the beginning of the trail to evaluate the HIV-RNA load in serum samples of infected subjects.
Clinical specimens
Blood samples collection and preparation: 10 cc's of blood was withdrawn and collected twice on the same day of examination. Blood specimens from all donors were collected by venipuncture followed by the anti-coagulation technique using K3EDTA (2 mg/ml). Plasma was separated from whole blood by centrifugation within 6 hrs of collection and was stored frozen at 270°C until tested. Three samples were taken, one before starting the trail, second after 12 weeks and the last one after 24 weeks.
Therapeutic protocol
Every patient in each group was advised to take the V20E immune Peptides combinations by different regimens were the group I took the combinations (F1,F2 and F3) in capsules form for three days continuously one capsule/day one hour after meal, repeating it after 45 days, the final dose after 90 days, group II took the combination in vial form for three days continuously as follow, one vial/day repeating it 45 days and the final dose after another 90, while the group III took the combination in capsule form for five days continuously as follow each patient advised to take one capsule/day repeating it every month for three successive months. Two Blood samples were collected from patients in every group at 12 and 24 weeks after finishing the V20E immune peptides treatment to investigate the results of these immunological data; Quantitative HIV-PCR, counting of CD4+ and CD8+ T-cell, free anti (gp120, p24 and RT ) and circulating immune-complex IC1, IC2, and IC3.
The immunological data among all studied groups before V20E treatment
The outcome measure revealed that patients of group I their immunological data showed viral load count (>500,000 copies/ ml), circulating free nAbs were (14.2-16.8 ng/ml) for anti gp120, (18.7-20.5 ng/ml) for anti P24 and (11.2-12.5 ng/ml) for anti-RT (detection ranges 0.03 to 0.05 ng/ml) as in (Figure 1), IC1 (1.1-2.1 ng/ml ), IC2 (0.9- 2.4 ng/ml) and IC3 (0.3-1.2 ng/ml) were their detection range (0.5-5 ng/ml) as in (Figure 2), CD4+ T-cell count more than (29-33%) and the CD8+ T-cells (60-64%) as expressed in (Figure 3,4 and 5), in group II their viral load were (<100,000 copies/ml), circulating free nAbs were (6.2-7.5 ng/ml) for anti gp120, (7.3-9.5 ng/ml) for anti P24 and (3.2-4.2 ng/ml) for anti-RT, IC1 ( 5.2-6.6 ng/ml), IC2 (2.7-4.6 ng/ml ) and IC3 (3.1-3.9 ng/ml), CD4+ T-cell count (20-27%) and CD8+ T-cells ranged from (59-69%) and in Group III the viral load recorded (<10,000 copies/ml), the levels of circulating free nAbs were (4.2-5.8 ng/ml) for anti gp120, (3.2-4.5 ng/ml) for anti P24 and (1.2-3.5 ng/ml) for anti-RT, IC1 was (14.7-16.0 ng/m), IC2 (12.1-13.2 ng/ml) and IC3 (9.8-10.5 ng/ml),CD4+ T-cell count (8-19%) and the CD8+ T-cells ranged from (70-82%). The control group showed viral load of (<40,000 copies/ml), free nAbs (5.5-5.8 ng/ml) for anti gp120, (6.0-6.5 ng/ml) for anti P24 and (3.2-3.8 ng/ml) for anti-RT, IC1 was (9.0-11.0 ng/m), IC2 (8.1-10.2 ng/ml) and IC3 (7.8-8.5 ng/ml), CD4+ T-cell count (18-28%) and CD8+ T-cells ranged from (55-81%).
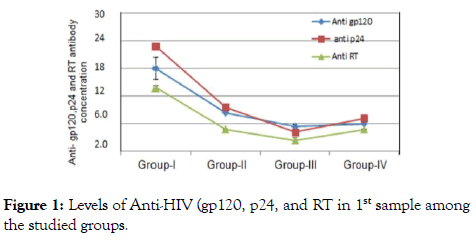
Figure 1. Levels of Anti-HIV (gp120, p24, and RT in 1st sample among the studied groups.
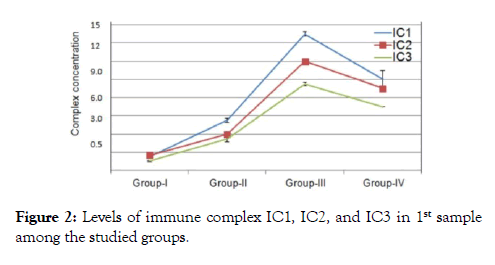
Figure 2. Levels of immune complex IC1, IC2, and IC3 in 1st sample among the studied groups.
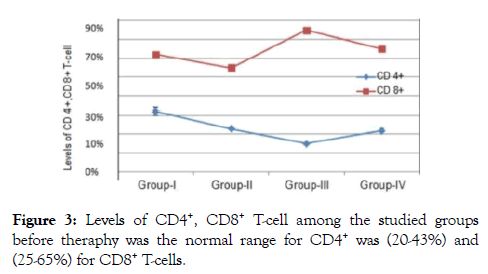
Figure 3. Levels of CD4+, CD8+ T-cell among the studied groups before theraphy was the normal range for CD4+ was (20-43%) and (25-65%) for CD8+ T-cells.
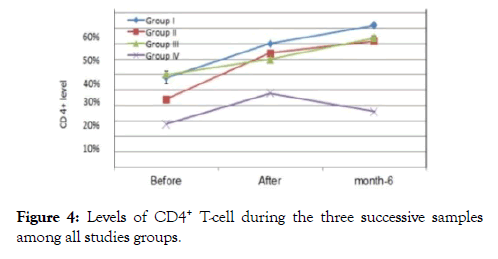
Figure 4. Levels of CD4+ T-cell during the three successive samples among all studies groups.
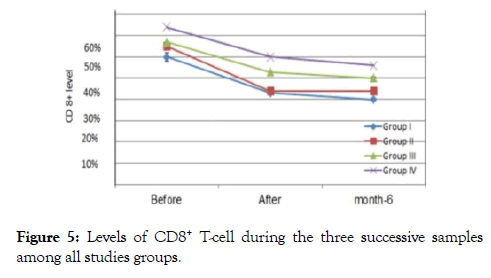
Figure 5. Levels of CD8+ T-cell during the three successive samples among all studies groups.
The immunological data among all studied groups 12, 24 weeks after (V20E) as well as the (ARVs) treatment protocol
All the collected blood samples assayed to measure the concentrations level of circulating complex (IC1, IC2, and IC3), count number of CD4+ and CD8+, Quantitative measuring of (HIV PCR-RNA) and the freely nAbs of (gp120, p24 and RT), 12 and 24 weeks after intake the novel combination therapy (V20E) as well as the (ARVs) revealed that The results of the second sample 12 weeks after finishing the three months therapy.
In group I after 12 weeks, about 4 patients showed undetectable viral loads, 3 patients showed a very mild viral titer (300-1100 copies /ml) as in (Figures 6-8), marked elevation of the CD4+ Tcells count ranged from (32-39%), reduce in count of the CD8+ T-cells to (42-52%), the concentrations of immune complex IC1, IC2, and IC3 showed two negative cases, the others 5 patients showed level from 0.2-1.1, while the concentration of anti (gp120, p24 and RT), five patients recorded negative results, the other two patients showed value from (0.5-1.5 ng/ml).
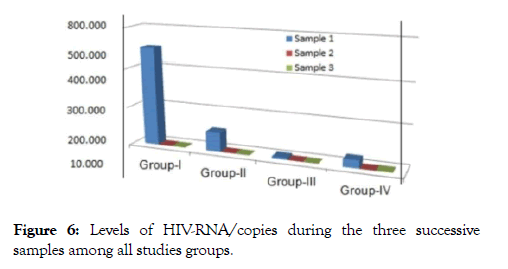
Figure 6. Levels of HIV-RNA/copies during the three successive samples among all studies groups.
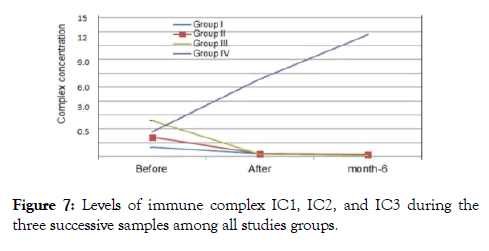
Figure 7. Levels of immune complex IC1, IC2, and IC3 during the three successive samples among all studies groups.
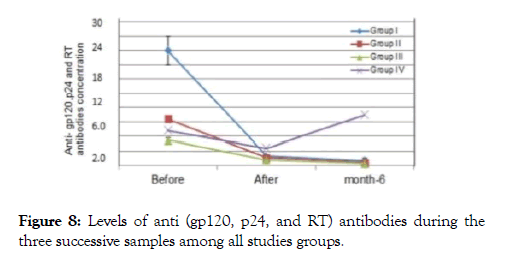
Figure 8. Levels of anti (gp120, p24, and RT) antibodies during the three successive samples among all studies groups.
In group II about 4 patients showed undetectable viral loads, 3 patients showed a very mild viral titer (1200-3100 copies/ml), marked elevation of the CD4+ T-cells count (33-41%), CD8+ Tcells were (43-52%), the concentrations of immune complex IC1, IC2, and IC3 showed three negative cases 4 patients showed level from 0.4-1.1, while anti (gp120, P24 and RT), six patients recorded negative results, one showed value from (0.2-1.0 ng/ml).
In group III about 6 patients showed undetectable viral loads, one patients showed a very mild viral titer (1100 copies /ml), In control group were all of them followed the regimen protocol under surveillance by HIV/AIDS Control Department at the Egyptian Ministry of Health (MOH) to take the Antiretroviral drugs (ARVs), three patients showed undetectable viral loads, one recorded (400 copies /ml), CD4+ T-cells count were (23-27%), CD8+ T-cells were (56-66%), concentrations of immune complex IC1, IC2, and IC3 showed level from 26.0-30.6, while the results of the free nAbs (gp120, P24 and RT) showed value from (2.2-3.8 ng/ml).
24 weeks after the end of the therapy in group I, only four patients from seven were come back again and accept to complete the trail, three of four showed undetectable viral loads, one recorded (249 copies/ml), CD4+ T-cells count reached to (46-52%), CD8+ T-cells to (34-52%), the concentrations of immune complex IC1, IC2, and IC3 showed one negative cases, three patients showed level from 0.2-1.0, the anti (gp120, p24 and RT) showed three negative results, one patient showed value from (0.1-0.7 ng/ml).
In group II, five patients were come back to complete the trail and the other two not came, four of five showed undetectable viral loads,one recorded (1100 copies /ml), CD4+ T-cells count reached to (37-48%), CD8+ T-cells to (45-55%), the concentrations of immune complex IC1, IC2 and IC3 showed three negative cases, one patients showed level from 0.4-1.0, anti (gp120, p24 and RT) showed four negative results, one patient showed value from (0.1-0.4 ng/ml).
In group III five patients were come back to complete the trail, two patients not came, were all of them showed undetectable viral loads, CD4+ T-cells count reached to (40-48%), CD8+ Tcells (45-58%), the concentrations of immune complex IC1, IC2, and IC3 showed four negative cases, one patients showed level from 0.2-0.4, anti (gp120, p24 and RT) four patients showed undetected value, one patient showed value from (0.1-0.6 ng/ml), two patients of the control group were came to complete the trail, their results were as follow; the two patients showed positive viral loads, ranged from (300-2300 copies /ml) CD4+ T-cells count were (18-22%), CD8+ T-cells were (60-61%), the concentrations of immune complex IC1, IC2, and IC3 showed level from 40.0-52.0, the free nAbs (gp120, P24 and RT) showed value from (6.2-9.2 ng/ml).
Understanding HIV mode of action and its strategy will reduce more researches efforts, and can open the gate for developing an effective vaccine or therapy that could help to reduce the severity of the disease and prevent the spreading of infection. Current therapy with Antiretroviral drugs (ARVs) interferes with the viral replication process. However they are very effective in reducing the amount of HIV level in patient’s blood, it cannot completely eradicate HIV, even if patients continue taking ARVs for several years, HIV will stay hiding in various parts of their body, known as “viral reservoirs”, by stopping or interrupting the course of therapy, HIV can re-establish itself by leaking out of these reservoirs [18,19]. All the previous reports still confused about the potential role of the HIV nAb which is generated against the viral antigens particles, if it has a protective or a pathogenic role in HIV infection?, depending on our assumes, formation of Immune complex IC1, IC2 and IC3 (HIV antigens and it’s specifically antibodies in bounded form) in serum sample of HIV infected patient, play the main role in protecting the HIVRNA from cytotoxic T-cells invading. To introduce a real cure for HIV we have to destruct or stop the formation of these immune complexes. In order to prove our postulation we formulated in vitro a different forms of immune complex as [F1, F2, and F3], and immunized the rabbits groups separately to stimulating its immune cells to eliciting antibodies for each specific [F1, F2, and F3] in a form of (anti F1, F2, and F3) antibodies. In screening new marker test was designed to catch the three complexes structure [IC1, IC2 and IC3] in serum sample of infected patients. Results collected from the first sample revealed that, all patients in the four groups have circulating immune complexes and also denoted to the strong correlation between; increasing in concentration measure of circulating immune-complex (IC1, IC2, and IC3), decreasing in viral load of serum HCV RNA, decreasing in the level of free neutralizing anti-HIV (gp120, P24 and RT antigen) antibody, on the other hand a correlation between, decreasing in the level of these immune-complex [IC1, IC2 and IC3], increasing in viral load of serum HCV RNA and the increasing in the level of free neutralizing anti-HIV (gp120, p24 and RT antigen) antibody, these results clearly explained our assumes and showed the role of bounded nAbs as well as the free nAbs in controlling and hiding the viral antigens. During the acute infection, the free broadly neutralizing antibodies are high, reflexing the inability of the viral antigens to attract the all produced antibodies, while in chronic state the viral antigens succeed in attracting most of the neutralizing antibodies causing a decrease in free Abs form and increase in bounded antibody form [IC1, IC2 and IC3], these results introduced an explanation for the main cause behind the increased level of the CD8+ T-cell during chronic infection, decrease in viral load and increase in level of immune complex [IC1, IC2 and IC3] where may be a result of CIC formation that stimulates the regeneration of more positive CD8+ T-cells than existed sick cells, in trying to counter act the virus, and the increased in the mutated CD4+ T-cells which became another CD8+ T-cells. VE 20 is an immune peptide was created to act as a novel therapeutically intervention for curing HIV by coupling (Ag/nonspecific Abs) in one form differs from the already existed CIC that share (Ag/specific Abs). The main aim for this novel immune peptide VE 20) is to stimulate the attention of CD8+ T-cell towards the other hidden circulating immune complex form (Ag/specific Abs), to put the CD8+ in state of confusion to distinguish between the existing CIC and the similar intruder noncomplex, finally the Cytotoxic choose to destruct both of them. In studying the results of the 2nd,3rd samples and comparing it with the results of the 1st sample, to evaluate the efficacy behind the using of our novel immune noncomplex (VE20 ), we found that all patients in groups [I,II and III] 75% of them showed undetectable viral loads, marked progress in count of CD4+ T-cells, diminishing in concentration levels of immune-complex [IC1, IC2 and IC3], decreasing in count of CD 8+ T-cells and undetected concentrations level for the free anti [gp120, p24 and RT], by comparing results of the 1st sample with the 2nd, 3rd samples of control group, we found that after 6 months therapy with (ARVs), 50% of the patients showed undetectable viral loads, increasing in concentration levels of the immune-complex [IC1, IC2 and IC3], positive detection for the free anti [gp120, P24 and RT] and mild progress in the count of CD 4+ T-cells but didn’t reach the normal or below level. In comparing the results of our novel therapy to the already existed therapy, we discovered that our new therapy succeeded in changing the all clinical signs and symptoms of the examined patients, by destructing the immune complex IC1, IC2, and IC3 which is used by HIV to cover and protect itself from being attacked by the cytotoxic CD 8+ T-cells, this destructive action leads to clearance of the viral RNA, also this novel therapy succeeded in stopping the CD4+ T-cells mutation and the infection of other healthy cells which finally leads CD4+ T-cells regeneration, controversial to the role of ARVs which succeeded in viral clearance in most of the patients and a slight increase in CD4+ T-cells count, but the levels of the immune-complex [IC1, IC2 and IC3] showed marked increase by starting the treatment with the ARVs, the clinical signs and symptoms still showing unsatisfying progress.
To identify the best regimen therapy where the end point of our study was to reach with treated patients to undetectable viral loads (less than 50 copies/ml), and increasing in count of CD4+ T-cells and clearance of the immune-complex [IC1, IC2 and IC3], we found the three regimen protocol has a high successful rates especially in group III, were their results was completely changed after 12 and 24 weeks, almost treated patients became undetectable HIV RNA and their CD4+, CD8+ T-cells reached to normal values. We completely convinced by the results of the study, in finding a positive correlation between the clearance of these immune-complex and the regeneration of the CD4+ T-cells as well as the eradication of HIV RNA from the all infected CD4+ T-cells. Also we are highly aware without any doubts that our results still need a sufficient evidence to use this newly discovered combinations peptide as therapeutic and prophylactic for treatment of HIV as well as we recommend extra studies to examine the role of circulating immune complex in viral suppression or activation through in vitro and in vivo studies and another experiments to validate the pathogenicity of these HIV-1 bnAbs but due to the lack of funding our studies weren’t completed. In conclusions these results raises the possibility of developing therapeutic and prophylactic treatment or vaccine as well as novel serological assays for monitoring HIV treatment.
We thank Dr. Nada for provision of immune-complex preparations and ELISA preparations; Dr. Haider Ghaleb designed and supervised the project. Dr. Sherif Salah performed the experiments and prepared the manuscript. The animal study was performed in accordance with regulation and guidelines of the Institutional Animal Care and Use Committee of the Cairo University.
All authors consent for publication and approved the manuscript.
None of the authors have any source of funding to declare.
The authors declare that they have no competing interests.
Citation: Sharif SH, Ghaleb HA, Sherif N (2019) HIV Immune Complex as a New Promising Vaccine Eliciting Broad Immune Response, Alternative to Conventional Vaccine. J Antivir Antiretrovir. 11:182. doi: 0.4172/1948-5964.1000182
Received: 14-Mar-2019 Accepted: 21-Mar-2019 Published: 28-Mar-2019
Copyright: © 2019 Sharif SH, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Sources of funding : GB´s PhD-project on ethical challenges and decision-making in nursing homes has been financially supported by the Norwegian Extra Foundation for Health and Rehabilitation through EXTRA funds (grant no. 2008/2/0208).