Fungal Genomics & Biology
Open Access
ISSN: 2165-8056
ISSN: 2165-8056
Review Article - (2022)Volume 12, Issue 2
Histidine kinases are protein kinases that are involved in signal transduction. Histidine kinases and the downstream the response regulators constitute a two-component phosphorelay system. In exposure to environmental stimuli, histidine kinase autophosphorylates, and the cytoplasmic response regulator component transmits the signal that activates transcription of multiple genes. In fungi, the histidine kinases are classified into 11 groups that are involved in various physiological processes, including virulence, conidition, morphogenesis, osmosensing, light sensing, and growth. In addition, phylogenetic analysis of 28 different fungal histidine kinases revealed that histidine kinases are evolutionary conserved.
Fungi; Histidine kinase; Neurospora crassa; Response regulator
Histidine Kinases (HKs) were first identified in prokaryotes in the early 1980s [1]. HKs were also recognized in the higher eukaryotes, including fungi and plants since 1990s [2,3]. HKs and the downstream target protein, also referred as Response Regulator (RR), form a two-component phosphorelay system. The Two-Component System (TCS) structure is generally characterized in two categories based upon whether the HK and RR domains present on same protein or different proteins [4]. In the simple TCS or Simple Histidine Kinase (SHK), both HK and RR domains are present on a different protein (Figure 1A) [5]. In the two-component phosphorelay, a complex variant of the TCS signaling pathway, the sensor is normally a hybrid HK (HHK) containing both HK and RR domains (Figure 1B). In TCS or SHK signaling, phosphate is directly transfers from histidine of the sensor to aspartate residue (His-to-Asp phosphotransfer) in the RR on separate protein [4]. In response to signal, the HHK or two-component phosphorelay, the sensor histidine is autophosphorylated and phosphate is transferred to the aspartate residue of the RR domain. Subsequent, another protein known as Histidine Phosphotransferase (HPt), phosphorylates and transfer phosphate to distinct response regulator, resulting in a His-Asp-His-Asp phosphorelay [4,6] (Figure 1B). Moreover, phosphorylated RR functions upstream of various cellular pathways, including, MAPK pathway, and activation of transcription factors [7,8] (Figure 2).
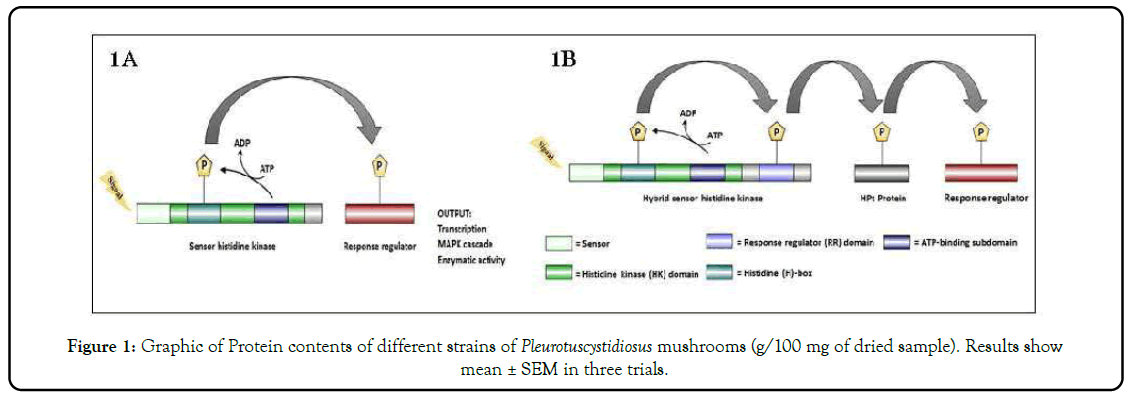
Figure 1: Graphic of Protein contents of different strains of Pleurotuscystidiosus mushrooms (g/100 mg of dried sample). Results show mean ± SEM in three trials.
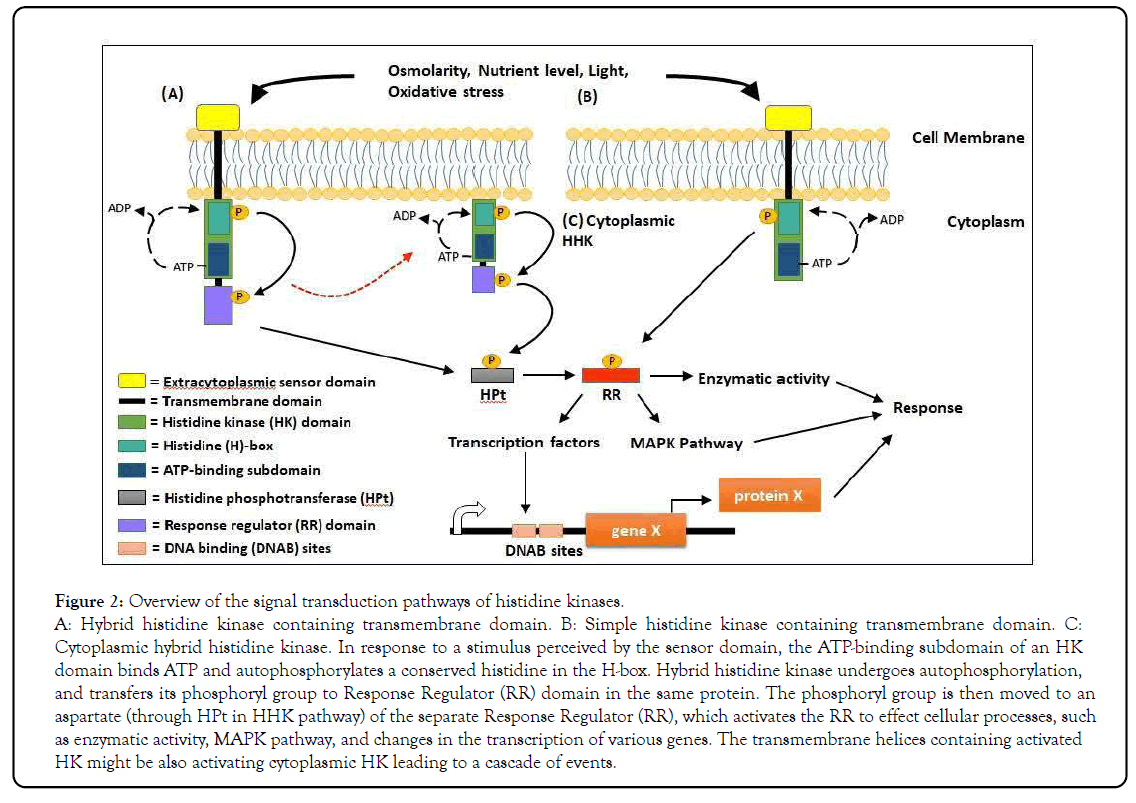
Figure 2: Overview of the signal transduction pathways of histidine kinases.
A: Hybrid histidine kinase containing transmembrane domain. B: Simple histidine kinase containing transmembrane domain. C: Cytoplasmic hybrid histidine kinase. In response to a stimulus perceived by the sensor domain, the ATP-binding subdomain of an HK domain binds ATP and autophosphorylates a conserved histidine in the H-box. Hybrid histidine kinase undergoes autophosphorylation, and transfers its phosphoryl group to Response Regulator (RR) domain in the same protein. The phosphoryl group is then moved to an aspartate (through HPt in HHK pathway) of the separate Response Regulator (RR), which activates the RR to effect cellular processes, such as enzymatic activity, MAPK pathway, and changes in the transcription of various genes. The transmembrane helices containing activated HK might be also activating cytoplasmic HK leading to a cascade of events.
Almost all eukaryotic HKs, including fungi, are hybrids, meaning that a single polypeptide comprises both the HK and RR domains [9,10]. The current availability of genome data for many fungi have made it easier to rapidly search for components of the HK signaling pathway and to investigate the diversity and uniqueness of the two-component phosphorelay. We performed phylogenetic analysis using the HK proteins sequences from N. crassa, M. oryzae, C. albicans, A. fumigatus, and M. grisea, which revealed that these proteins are highly conserved, and HKs are involved in diverse physiological processes.
Histidine kinase
The basic structure of the HK mainly consists of three main regions, including a highly variable N-terminal sensor domain that is responsible for specific stimulus recognized by the HK, a central region consisting of HK domain for dimerization or phosphoacceptor and ATP-binding subdomain or histidine kinase-like ATPase catalytic subdomain (HATPase_c), and the C-terminal Response Regulator (RR) that is characterized by the presence of phosphorylatable aspartate residue [11-13]. The HK domain includes an H-box that generally contains a phosphorylatable histidine residue, and an X-box [12]. The ATP- binding subdomain exhibits four distinct boxes, including N-, G1-, F-, and G2-boxes [11-15]. N-box comprises the conserved asparagine residue which interacts with the phosphate group of the ATP through a bound Mg2+ ion. G1-box contained a conserved aspartate residue which interacts with the N6 atom of ATP via salt bridges. Phenylalanine residue housed inside the F-box and aspartate residues comprised in the G2-box, these both boxes responsible for the positioning of adenine [16].
In bacteria, HKs are involved in diverse processes, including virulence, nutrient acquisition, development, quorum sensing, antibiotic resistance, and spore dormancy [17-19]. Eukaryotic HKs have possibly evolved from a single prokaryotic source by lateral gene transfer from bacteria [10,20]. In plants, HKs play a role in response to osmosensitivity, salt stress, ethylene, and for stomatal closure [2,17]. In rats and humans, the mammalian HKs Nucleoside Diphosphate Kinase (NDPK) and histone H4 histidine kinase have been characterized [21,22]. NDPK phosphorylates ATP-citrate lyase and succinate thiokinase [22-24]. Additionally, a recent study has demonstrated the role of Non- Metastatic Cells (NME) protein family member NME1/NDPK HK in neuroblastoma cell migration and differentiation [25]. In rat liver, H4 histidine kinase is responsible for phosphorylation of two histidine residues in the histone H4, which is an important step in the regeneration of liver after partial hepatectomy [22]. In fungi, HKs have been evolved to sense air, light, and host surface characteristics necessary for adequate growth and survival [26]. However, a recent study conducted by Bourret and his colleague showed that most hybrid HK do not engages in two component phosphorelay signaling in fungi [27]. The filamentous fungus N. crassa possess 11 HK genes [28]. In dimorophic fungi C. albicans, three HK genes have been identified [7]. In case of A. fumigatus, three out of previously identified 13 HKs were characterized [29]. The genome analysis of M. oryzae (Pyricularia oryzae) revealed 10 HK encoding genes [7,30,31].
Histidine phosphotransferase
Histidine Phosphotransferase (HPt) protein plays an important role in signal transduction. HPt acts as an intermediate in two- component phosphorelay signaling in eukaryotes. It comprised a conserved histidine residue, through which it mediates the phosphate transfer between sensor histidine kinase and distinct response regulator [6]. It allows eukaryotic organisms to expand histidine kinase signal transduction system beyond simple two-component system to highly complex two-component phosphorelay system [32]. HPt is highly conserved across eukaryotic species [33]. The genome of most of the fungi such as Saccharomyces cerevisiae, N. crassa, human pathogenic fungi C. albicans and A. fumigatus, and rice blast fungus M. oryzae encode one putative HPt gene that is homolog to the Saccharomyces cerevisiae Ypd1 [29,33-35]. In contrast, the genome of plants has more than one HPt gene [35]. In M. oryzae, the MoYpd1 gene, which undergoes alternative splicing to produce different isoforms of Moypd1 protein, may increase the signal diversity [36].
In N. crassa, HPt-1 is essential for growth, and it negatively regulates the MAPK cascade pathway [34]. In M. oryzae, MoYpd1 plays a significant role in conidiation by regulation of light responsive gene [35]. Further, loss of function of MoYpd1 results to a complete absence of conidiation [37]. The C. albicans ∆Ypd1 mutant is viable [33]. However, in Saccharomyces cerevisiae, Ypd1 (ScYpd1) is essential for viability [38].
Response Regulator
Response Regulator (RR) protein forms the second component of two component system in bacteria and phosphorelay signaling system in fungi and plants. The RR is the critical component to accomplish the specific cellular output in response to the stimulus received by the sensor HK [39]. RR protein comprised a conserved receiver (REC) domain at their N-terminals and effector domain at C-terminals [39,40]. During stimulus, REC domain catalyzes the phosphate transfer from its cognate HK or HPt to its well conserved aspartate residue. Upon phosphorylation, REC domain undergoes different conformational changes and effector domain in response to conformational changes produces an output response [41].
According to prokaryotic TCS database, prokaryotes genome contains gene for more than 80,000 RR [42]. Further, around 70% of the categorized prokaryotic RR contains a DNA-binding domain and acts as transcription factors [39] (Figure 2). In the bacterium Mycobacterium tuberculosis, MtrA response regulator, control the transcription of genes responsible for cell division and the cell wall integrity through binding to DNA [43]. In another bacterium Francisella novicida, response regulator BfpR acts as positive regulator of antimicrobial peptide resistance, and negatively regulates the biofilm formation [44].
On the other hand, Ascomycetes fungi have few numbers of RR such as N. crassa (RRG-1 and RRG-2), M. oryzae (MoSkn7 and MoSsk1), A. fumigatus (AfSskA and AfSkn7), and C. albicans (CaSsk1 and CaSkn7). The RRs in these fungi are homolog to Saccharomyces cerevisiae Ssk1p and Skn7p [8,29,34,45,46]. In N. crassa, RRG-1 response regulator regulates the MAPK pathway and controls hyperosmotic susceptibility, vegetative cell integrity, fungicide tolerance, and protoperithecial development [8] (Figure 2). In contrast, RRG-2 is not essential for fertility [34]. However, sensitivity to the oxidative stress increased in N. crassa mutant for RRG-2 gene. Further, a recent study has revealed that AfSkn7 involved in the pathway, responsible for increasing the chitin content in the cell wall in response to fludioxonil [47]. In the candida species C. auris, Ssk1 along with the MAPK Hog1 responsible for cell wall integrity and antifungal drug resistance. Moreover, deletion of Ssk1 in C. auris restores the antifungal susceptibility to caspofungin (CAS) and amphotericin B (AMB) [48]. In another plant pathogenic fungi Verticillium dahlia, RR VdSsk1 has role in Virulence, melanin biosynthesis, and oxidative stress. Further, V. dahlia mutant for VdSsk1 showed resistance to iprodione and fludioxonil [49].
Fungal histidine kinases contain conserved domains
We retrieved 28 fungal HKs amino acid (aa) sequences having a conserved HK domain (dimerization/phosphoacceptor; PS50109) and RR domain (PS50110; https://prosite.expasy.org/ scanprosite/, ScanProsite database expasy) from UniProtKB, then performed multiple sequence analysis (MSA) and phylogenetic analysis which revealed that these HKs belong to nine different groups (Figures 3 and 5) [50]. Furthermore, we used the ScanProsite database to predict the domain in HK proteins, which revealed additional signaling domains other than the conserved HK and RR domains. The additional domains are PAC- (motif C-terminal to PAS- motifs; PS50113), PAS- (period circadian protein, aryl hydrocarbon receptor nuclear translocator protein, single-minded protein; PS50112), PK- (protein kinase domain; PS50011), HAMP- (histidine kinase, adenylyl cyclase, methyl-accepting protein, and phosphatase; PS50885), and PHY-2 -(phytochrome region 2; PS50046) domains (Figures 4A-4I) [51]. However, among the 28 HK proteins analyzed, we could identify transmembrane helices only in four proteins that are members of the group VI (Figure 4E). Only one N-terminal Transmembrane (TM) helix was predicted in the sequence of N. crassa Sln1 (EAA29622.1) and M. oryzae Sln1 (EHA55738.1), three in C. albicans Sln1 (Q5A872.1), and two TM helices were predicted in A. fumigatus Sln1 (EBA27302.1). Except for these four HK proteins containing TM helices, all others appeared to be in the cytosolic or aligned with the membrane.
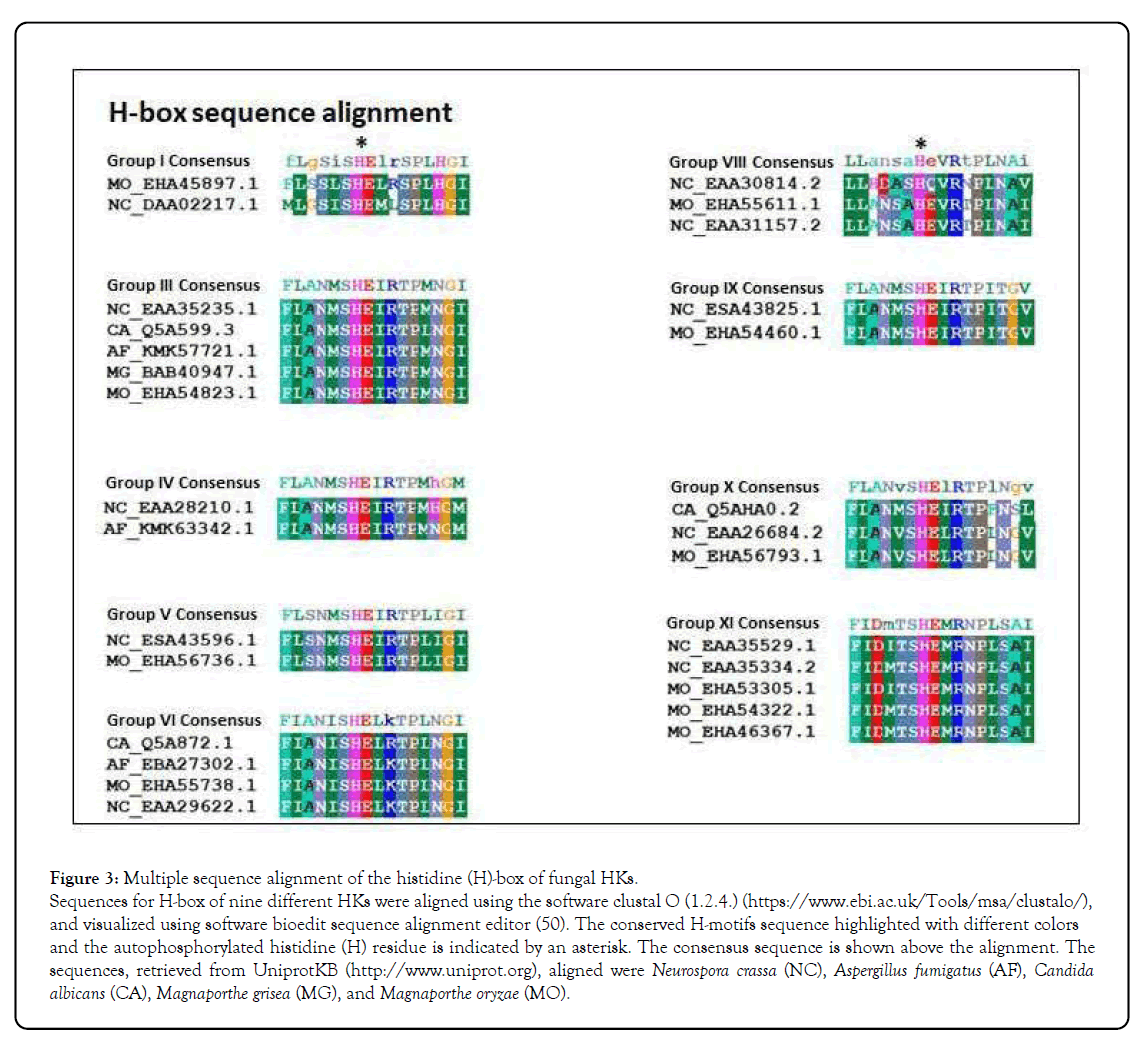
Figure 3: Multiple sequence alignment of the histidine (H)-box of fungal HKs.
Sequences for H-box of nine different HKs were aligned using the software clustal O (1.2.4.) (https://www.ebi.ac.uk/Tools/msa/clustalo/), and visualized using software bioedit sequence alignment editor (50). The conserved H-motifs sequence highlighted with different colors and the autophosphorylated histidine (H) residue is indicated by an asterisk. The consensus sequence is shown above the alignment. The sequences, retrieved from UniprotKB (http://www.uniprot.org), aligned were Neurospora crassa (NC), Aspergillus fumigatus (AF), Candida albicans (CA), Magnaporthe grisea (MG), and Magnaporthe oryzae (MO).
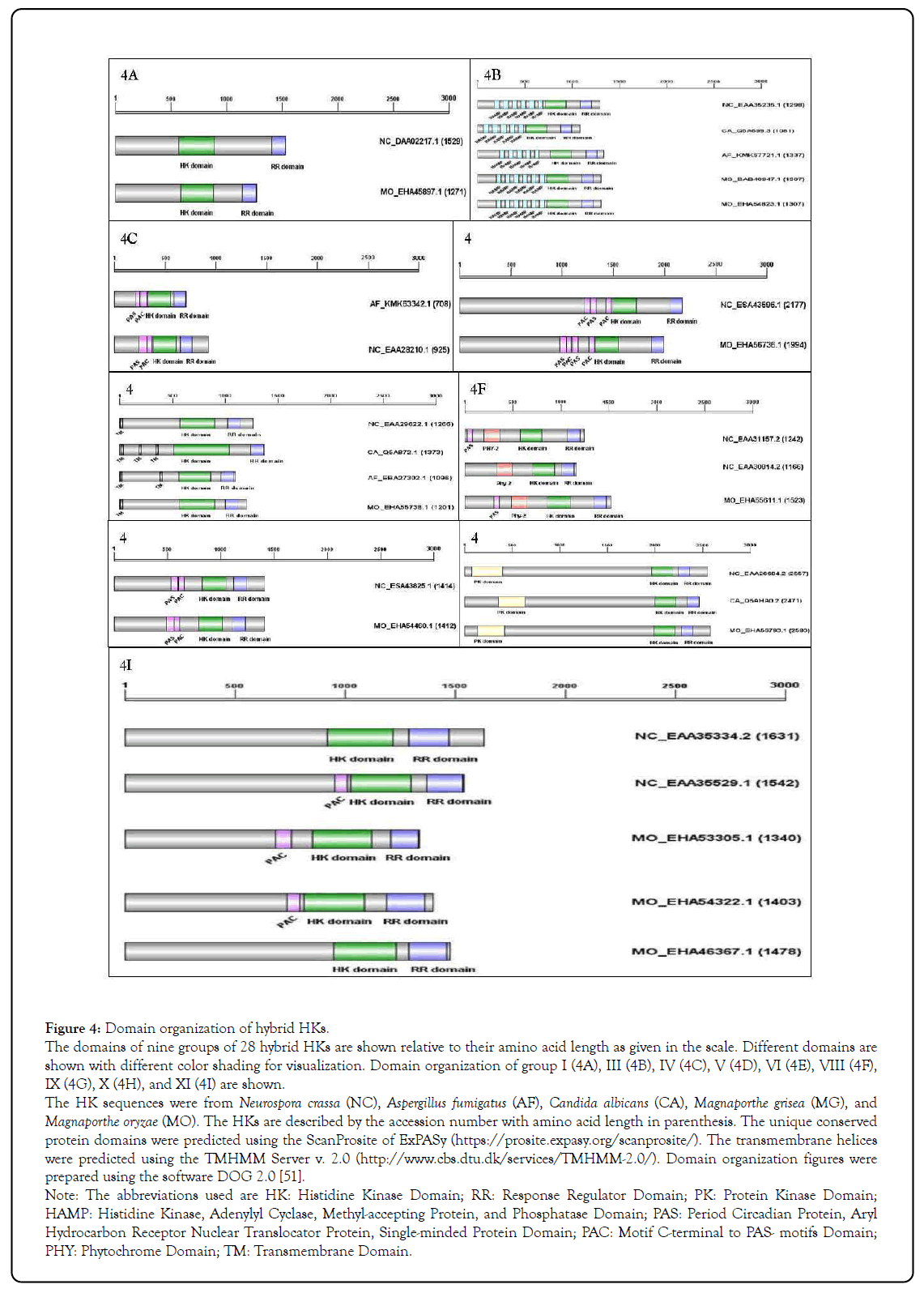
Figure 4: Domain organization of hybrid HKs.
The domains of nine groups of 28 hybrid HKs are shown relative to their amino acid length as given in the scale. Different domains are shown with different color shading for visualization. Domain organization of group I (4A), III (4B), IV (4C), V (4D), VI (4E), VIII (4F), IX (4G), X (4H), and XI (4I) are shown.
The HK sequences were from Neurospora crassa (NC), Aspergillus fumigatus (AF), Candida albicans (CA), Magnaporthe grisea (MG), and Magnaporthe oryzae (MO). The HKs are described by the accession number with amino acid length in parenthesis. The unique conserved protein domains were predicted using the ScanProsite of ExPASy (https://prosite.expasy.org/scanprosite/). The transmembrane helices were predicted using the TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM-2.0/). Domain organization figures were prepared using the software DOG 2.0 [51].
Note: The abbreviations used are HK: Histidine Kinase Domain; RR: Response Regulator Domain; PK: Protein Kinase Domain; HAMP: Histidine Kinase, Adenylyl Cyclase, Methyl-accepting Protein, and Phosphatase Domain; PAS: Period Circadian Protein, Aryl Hydrocarbon Receptor Nuclear Translocator Protein, Single-minded Protein Domain; PAC: Motif C-terminal to PAS- motifs Domain; PHY: Phytochrome Domain; TM: Transmembrane Domain.
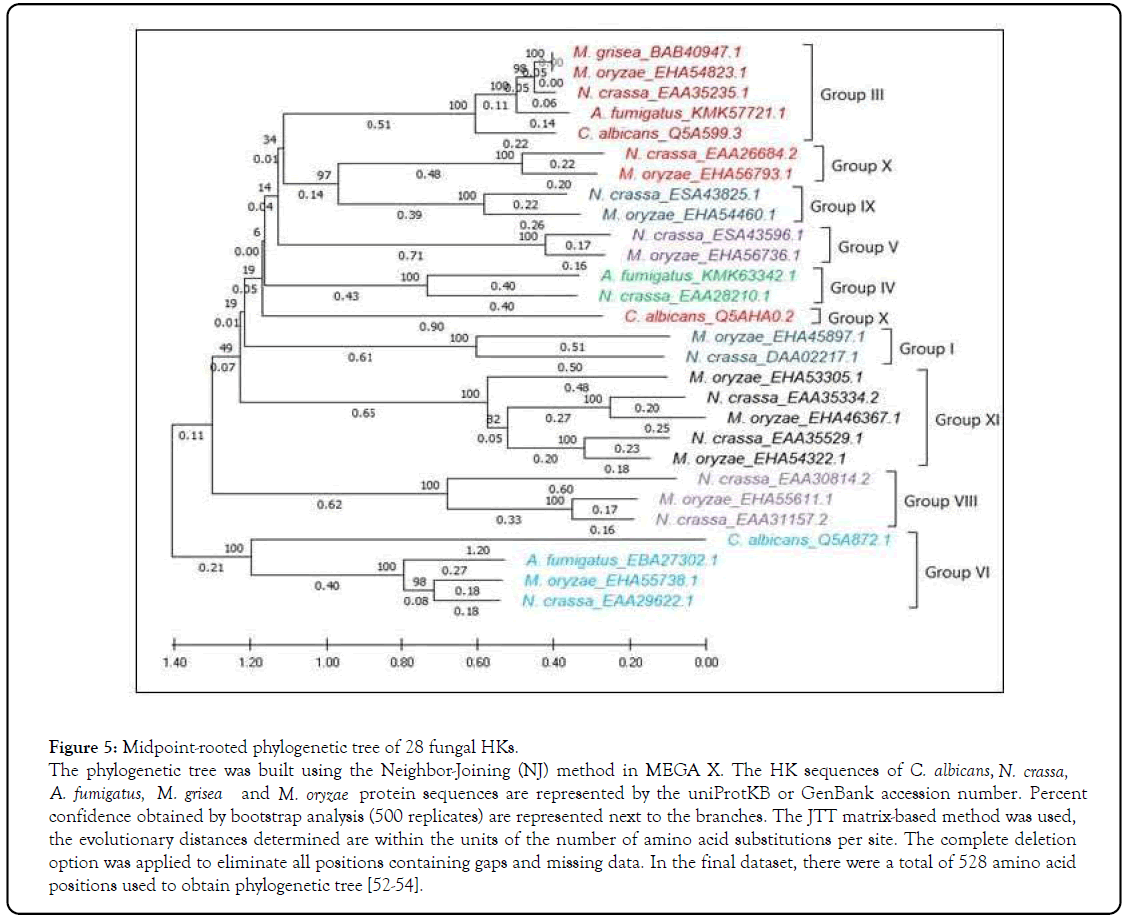
Figure 5: Midpoint-rooted phylogenetic tree of 28 fungal HKs.
The phylogenetic tree was built using the Neighbor-Joining (NJ) method in MEGA X. The HK sequences of C. albicans, N. crassa, A. fumigatus, M. grisea and M. oryzae protein sequences are represented by the uniProtKB or GenBank accession number. Percent confidence obtained by bootstrap analysis (500 replicates) are represented next to the branches. The JTT matrix-based method was used, the evolutionary distances determined are within the units of the number of amino acid substitutions per site. The complete deletion option was applied to eliminate all positions containing gaps and missing data. In the final dataset, there were a total of 528 amino acid positions used to obtain phylogenetic tree [52-54].
Phylogenetic analysis revealed that the fungal histidine kinases are closely related
In fungi, two-component sensor hybrid HKs were classified into 11 groups [7]. Phylogenetic analysis of the 11 sensors HK proteins from N. crassa (Sordariomycetes), and 10 HKs from M. oryzae (Sordariomycetes), three HK from each C. albicans (Saccharomycetes) and A. fumigatus (Eurotiomycetes), and one HK from M. grisea (Sordariomycete) using the Neighbor-Joining (NJ) method revealed 9 main groups and classified at least one member of HK from N. crassa in every group (I-XI), except for groups II and VII (Figure 5) [52-54]. HKs are highly conserved in filamentous ascomycetes, however, some HKs are very diverse and few clear orthologs have been identified [7]. HK groups III and VI, except M. grisea, encompass closely related sequences from each ascomycete fungi studied. Therefore, the sequences within group can serve as an orthologs with similar functions (Figure 5).
The group I HK present in the Basidiomycota, Ascomycota, and in early diverging fungi. Additionally, group I HK seems to have been expanded in the pathogenic ascomycota. Only one member from group I lacking N-terminal domain is found in N. crassa (DAA02217.1) and M. oryzae (EHA45897.1) (Figure 4A). In M. oryzae, mutants lacking Group I HK exhibit reduced in attenuated virulence and conidiation [31,55].
The groups III HKs are well-studied among all the HK groups studied. All group III members contain the conserved N-terminal region with multiple HAMP domains (PS50885) (Figure 4B). The N. crassa HK shows 85% identity with the HKs of M. oryzae and M. grisea, 68% with A. fumigatus and, and 49% with C. albicans. In fungi, group III HKs were only identified in terrestrial species, which implies that these sensing proteins were evolved during the process of land colonization [13]. Besides Sln1 HK (group VI), group III HKs (NIK1 orthologs) are shown to associate with os (osmosensitive) signal transduction pathway and are necessary for adaptation to high osmolarity conditions in different species of fungi [56]. They also regulate sporulation, morphogenesis, and virulence factor expression in plant- and animal-pathogenic fungi [57]. Some studies have suggested that the group III HKs is involved in fludioxonil and iprodione mediated antifungal action [58-60]. Additionally, in the plant pathogenic fungus Cochliobolus heterostrophus, Dic1 (Homologues to group III HK of N. crassa) mutant shows sensitivity to osmostic stress and resistance to fungicides, including fludioxonil and iprodione [61]. In C. albicans, NiK1 activates the Hog1 Stress-activated protein kinase (SAPK), which further promotes fungicidal activity, in response to distinct antifungals [62]. A recent study showed that heterologous expression of Blastomyces dermatitidis group III HK, dimorphism- regulating kinase 1 (Drk1), in S. cerevisiae and upon exposure of S. cerevisiae to fludioxonil modifies the activity of Triosephosphate Isomerase (TPI) [63]. Further, modified TPI causes the release of Methylglyoxal (MG), an aldehydic stress, into cytosol of S. cerevisiae [63]. Moreover, elevated MG level in cytosol, set off the transformation of heterologous expressed Drk1 kinase activity to phosphatase activity [64]. Subsequently, Drk1 dephosphorylates RR Ypd1 to constitutively turn on the HOG signaling which leads to S. cerevisiae cell death [63,64]. Group III HK TcsC in A. fumigatus and NikA in A. nidulans required for wild type growth, fludioxonil mediated cell wall reorganization, and resistance to oxidative stress [65].
The N-terminal domain of group IV HKs contains PAS/PAC domains (PS50112/PS50113) (Figure 4C). N. crassa contains one member of this group, EAA28210.1. An ortholog of EAA28210.1 in A. fumigatus is KMK63342.1, which shares a common ancestor with 100% bootstrap value (Figure 5). In a murine model of systemic aspergillosis, mutant lacking KMK63342.1 showed a substantial decrease in virulence [66]. The ortholog of N. crassa group IV is not identified in M. oryzae. Group V HK contains two sets of PAS/PAC domains (PS50112/PS50113) at their N-terminal (Figure 4D). Group V might be evolved by duplication and divergence, gaining an extra set of PAS/PAC domains. In a rice infection model study, M. oryzae mutant strain for the corresponding HK (MoHik5) showed decreased vegetative growth, hyper-susceptibility to stress, abolished virulence, cell wall instability, and lack of conidiation [31].
Phylogenetic relationships among HKs showed that group VI HKs is most divergent among all 9 HK groups (Figure 5). In the group VI HKs, N. crassa Sln1 (EAA29622.1) is more closely related to M. oryzae Sln1 (EHA55738.1) and distantly related to C. albicans Sln1 (Q5A872.1) (Figure 5). In C. albicans, the Sln1 HK regulates yeast to hyphae morphogenetic switch, osmotic adaptation, and virulence [67]. In Saccharomyces cerevisiae, Sln1 regulates the HOG1 MAP Kinase pathway in response to changes in external osmolarity conditions [38]. In a study using rice infection model, Sln1 was found associated with virulence, stress adaptation, cell wall stability, and conidiation [31]. However, in A. fumigatus, Sln1 (tcsB) gene disruption did not contribute to major changes in phenotype [56,68]. In A. nidulans, the group VI HK TcsB/Sln1 along with another protein phytochrome FphA is involved in temperature sensing and induced temperatures responsive gene expression [69].
Group VIII HKs contain an N-terminal phytochrome domain (Figure 4F), which is primarily involved in light sensing and vegetative growth [70-72]. The group VIII HKs also modulates light-dependent sexual development in N. crassa [73]. Two phytochrome-related HK in N. crassa (EAA31157.2, and EAA30814.2), and one in M. oryzae (EHA55611.1) were predicted. The N. crassa phytochrome EAA31157.2 and the M. oryzae phytochrome.
EHA55611.1 have a PAS and a PHY domains like fungal phytochrome orthologues. Therefore, the extra phytochrome- like HKs might be the results of duplication and divergence or, less likely, the results of selective loss of the PAS domain from a common ancestor (Figures 4 and 5).
Moreover, the phylogenetic analysis revealed that the group IX and X clades share a recent common ancestor (Figure 5). The HKs in the group X, which is highly divergent among all the HK groups, includes the member from N. crassa (EAA26684.2), M. oryzae (EHA56793.1), and C. albicans (Q5AHA0.2). In addition to the N terminal to the conserved HK and RR domains, these HKs contain other domains, including PAS/PAC (PS50112/PS50113) domains in the group IX, and protein kinase (PK) domain (PS50011) in the group X, (Figures 4G and 4H). The C. albicans CaHK1 (Q5AHA0.2) shares PK, HK, and RR domains with the N. crassa and M. oryzae orthologs, but does not share a common ancestor with them (Figure 4). Group IX HKs are responsible for virulence in M. oryzae, but do not affect the overall development of these fungi [31]. Moreover group X HKs were found associated with the cell wall biosynthesis, fungicide resistance, spore germination, attenuated virulence, and sensitivity to oxidative stress [31,45,74]. In another candida species C. guilliermondii, CHK1 (group X HK) does not participate in stress tolerance, cell wall composition, drug susceptibility and growth [75].
Group XI comprises cytoplasmic HKs, contain PAC (PS50113) domain along with the conserved HK and RR domains (Figure 4I). In N. crassa, the Group XI HK DCC-1 (EAA35529.1) is involved in the regulation of conidiation, vegetative growth, perithecial development, and carotenogenesis [76]. In M. oryzae, knockout mutation of EHA46367.1 showed reduced conidiation and abolishes appressorium development [77].
HKs are primarily involved in various pathways including, conidiation, fungicides resistance, osmosensing, light sensing, and growth in fungi. We also performed multiple sequence alignment and phylogenetic analysis of 28 fungal HK proteins. Among all the HK groups, only group VI HKs contain the transmembrane domain, and they regulate the downstream MAPK pathway in response to the external osmolarity conditions. The group I HKs lack N-terminal domain. To date very few HKs have been characterized in fungi, and functions of the HKs are remained largely unknown. In future, detailed functional characterization of these fungal HKs would help in understanding the cellular role of each of the HKs in fungi.
We acknowledge all the researchers in the relevant field for the contributions, which helped us writing this review article. We also regret our inability to cite all the work. MK is supported by a fellowship from MHRD. We also acknowledge the M. Tech. grant HoD/BT/01, Department of Biotechnology (DBT), Govt. of India. The RT laboratory is supported by a DBT-NER twinning grant BT/PR24473/NER/95/737/2017, from DBT, Govt. of India.
[Crossref]
[Crossref]
Citation: Kumar M, Tamuli R (2022) Histidine Kinases Play Diverse Cell Functions In Fungi. Fungal Genom Biol. 12:183.
Received: 21-Jan-2022, Manuscript No. FGB-22-15564; Editor assigned: 25-Jan-2022, Pre QC No. FGB-22-15564 (PQ); Reviewed: 08-Feb-2022, QC No. FGB-22-15564; Revised: 14-Feb-2022, Manuscript No. FGB-22-15564 (R); Published: 21-Feb-2022 , DOI: 10.35841/2165-8056.22.12.183
Copyright: © 2022 Kumar M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.