Journal of Applied Pharmacy
Open Access
ISSN: 1920-4159
ISSN: 1920-4159
Research Article - (2021)Volume 13, Issue 7
Objective: Aim of the current investigation is to screen the hepatoprotective activity of the Ethanolic Extract of Allophylus Cobbe (EEAC) leaves against Paracetamol (PCM) induced hepatotoxicity in rats.
Methods: EEAC was administered to the rats for 7 days (200 mg/kg and 400 mg/kg) and hepatotoxicity was induced by administration of PCM (3 g/k day . After 24 h of toxicity induction, the blood samples were collected by retro orbital plexus method and different serum and tissue biochemical parameters were analyzed. Livers of the animals were isolated and were studied for histopathological changes. The extract treated animals were compared with Silymarin (100 mg/kg).
Results: Treatment of animals with EEAC at 200 and 400 mg/kg showed dose dependant significant decrease in Serum Glutamate Oxaloacetate Transaminase (SGOT), Serum Glutamate Pyruvate Transaminase (SGPT), Alkaline Phosphatase (ALP), Acid Phosphatase (ACP), Creatinine and Lipid Peroxidation (LPO) levels and significant increase in protective enzymes Superoxide Dismutase (SOD), Catalase (CAT), Glutathione (GSH) and total proteins levels in serum and tissue biochemical parameters when compared with the PCM treated rats. In histopathological study of liver, animals pretreated with EEAC showed minimal liver damage with distinct protection of structures and the architectural frame of the hepatic cells as observed by the liver section showing mild degree of liver damage, sinusoidal congestion, and mild inflammation. Allophylus Cobbe showed the presence of bioactive components having antioxidant potential; which might be responsible for hepatoprotective activity of the EEAC.
Conclusion: The present study showed that EEAC restored the levels of altered biochemical parameters and prevented the liver from the toxic effects of PCM concludes that Allophylus Cobbe possess hepatoprotective activity.
Hepatoprotective activity; Silymarin; Paracetamol; Allophylus cobbe; Hepatotoxicity
The morphological and functional integrity of the liver is important to the health and human organism. It is dependent on constant maintenance of the various biochemical functions of the liver and the metabolic processes occurring in the hepatocytes and the sinusoidal cells. Human liver is known for different major metabolic functions for example, metabolism of bilirubin, porphyrin, bile acids, amino acids, proteins, carbohydrates, lipids, hormones, vitamins, biotransformation and detoxification functions, alcohol degradation, acid-base balance and so on. Thus liver is the important organ required to maintain the body’s homeostasis [1]. Worldwide, over the last several years, liver diseases have been increased enormously to become one of the leading causes of death and illness. According to the global burden of liver disease, Liver disease accounts for approximately 2 million deaths per year worldwide, which are due to cirrhosis, viral hepatitis and hepatocellular carcinoma. Recently cirrhosis is the 11th most common cause of death and liver cancer is the 16th leading cause of death; collectively, they account for 3.5% of all deaths globally [2].
PCM is the most commonly used analgesic worldwide and recommended as first-line treatment in all pain conditions by WHO [3]. It is also used for its antipyretic effects, helping to reduce fever. PCM induced hepatotoxicity is well established experimental model to determine the hepatoprotective activity of new pharmacological agents [4]. Therapeutic dose of PCM is considered safe for therapy but at higher doses, PCM can cause centrilobular necrosis that eventually leads to liver failure. The major advantage of PCM model is that it is a clinically relevant model and is a dose dependent hepatotoxicant.
At therapeutic doses, PCM is metabolized by glucoronidation or sulfation by cytochrome P450 system and the excess of it get converted into the reactive metabolite N-Acetyl-P-benzoquinone Imine (NAPQI) [5]. Under normal condition, NAPQI is the bioconverted into non-toxic metabolites by the enzyme glutathione (GSH). However, in case of overdose, excess NAPQI depletes GSH content and binds covalently to hepatic cellular proteins resulting in mitochondrial dysfunction and mitochondrial oxidative stress that eventually induces necrosis and apoptosis of hepatocytes [6]. Thus PCM-induced hepatotoxicity has been studied for several years due to their detrimental effects on health.
Allophylus Cobbe is a small shrub tree from the family Sapindaceae commonly known as Tippani in Marathi grows up to 5 m. Allophylus Cobbe has strong ethnobotanical and ethnopharmacological background. The bark is bitter, sweet and astringent. It has digestive, carminative, constipating and antiinflammatory properties. It is useful in ulcers, wounds, dyspepsia, anorexia, diarrhea, stomachache, fever, bruises and inflammation. In Konkan region the bark is used in bone fractures and dislocation of joints. The leaf extract is taken against stomachache and leaf pest is applied on scabies. The root power mixed with honey is a remedy for diarrhea whereas leaf juice effectively combats the ulcers. The fruits are cooling, sweet and tonic and are advised in general debility [7]. Amid these enormous medicinal values, the current investigation is aimed to investigate the hepatoprotective activity of Allophylus Cobbe in PCM-induced hepatotoxicity rat model.
Plant material collection preparation of plant extract
The leaves of the Allophylus Cobbe were collected from the forest of Jyotiba, Kolhapur (District), Maharashtra, India. Leaves were identified and authenticated by the Botanical Survey of India, Pune with specimen voucher number- BSI/WRC/ IDEN.CER./2018/H3-69-SDC 02 of the plant deposited at the same. The leaves were shade dried for a week and finely powered using the blender. The powder was passed through the 100 mesh sieve size and stored in sealed polythene bags. The powered drug (100 g) was extracted with 500 ml ethanol for more than 6 hr by using the soxhlet extraction assembly and concentrated by rotary evaporation and vacuum drying. The yield of plant was recorded (4.5%) and stored at -20°C until they further use [8].
Chemicals
PCM and Silymarin were obtained from E Merck (India) Ltd. Mumbai and Ranbaxy laboratories Ltd. Baddi, H.P., respectively. Serum Glutamate Oxaloacetate Transaminase (SGOT), Serum Glutamate Pyruvate Transaminase (SGPT), Alkaline Phosphatase (ALP), Acid Phosphatase (ACP), and Creatinine were estimated by using diagnostic kit obtained from Pathozyme Diagnostic Ltd., Kagal, Kolhapur and Medsource Ozone Biomedicals Pvt. Ltd. All reagents were of analytical grade [9].
Maintenance of animals and their feeding
Albino Wistar rats of either sex (150-180 g) were obtained from Crystal Biological Solutions, Uruli Devachi, Pune Maharashtra. Rats were housed in standard polypropylene cages (3 animals per cage) and were maintained under standard hygienic conditions at 25-28°C with 12 hr light/dark cycle and provided with standard pallet diet procured from Pranav Agro, Sangli. Water and food was given ad libitum throughout the study. The animals were cared for and maintained as per the approved guidelines of the “Committee for the Purpose of Control and Supervision of Experiments on Animals” (CPCSEA, India) and the protocol was approved by the Institutional animal Ethical Committee, Rajarambapu college of Pharmacy, Kasegaon
Preparation of doses and treatments
The PCM was given to animals at dose of 3 g/kg orally. Aqueous suspension of EEAC was prepared in distilled water and different doses of extract (200 mg/kg and 400 mg/kg) and silymarin 100 mg/kg were given to animals orally.
Acute oral toxicity
The acute oral toxicity was performed as per the OECD set guidelines, revised draft guidelines 423, received from CPCSEA, Ministry of Social Justice and Empowerment, Govt. of India.
Evaluation of hepatoprotective activity
Treatment groups: Albino wistar rats of either sex weighing 150-180 g were randomly divided into 05 groups of six animals each for the determination of pharmacological activity by biochemical and histopathological parameters.
Group 1: Served as negative control and animals were given distilled water for 7 days.
Group 2: Served as positive control to which hepatotoxicity was induced by using PCM. The animals were given distilled water for 7 days and given PCM single dose (3 g/kg b.w.) orally on day 8.
Group 3: This was given the standard drug Silymarin at a dose of 100 mg/kg p.o. for 7 days (p.o.) followed by a single dose of PCM on day 8.
Groups 4: This treated with 200 mg/kg and 400 mg/kg of ethanol extract of Allophylus Cobbe for 7 days (p.o) followed by a single dose of PCM on day 8.
After the study period, the animals were sacrificed using ether anesthesia. Blood samples were collected by the retro orbital plexus and the serum was separated for evaluating biochemical parameters [10]. The liver was immediately removed and small as fraction was homogenized for tissue biochemical assay and small pieces were fixed in 10% formalin for histopathological assessment.
Blood biochemical assay
The biochemical parameters level of hepato specific marker enzymes were estimated by reported methods and a SGOT and SGPT by Modified Reitman and Frankel’s method , ALP by Modified Kind and King's method, ACP by Modified King's Method and creatinine by Modified Jaff’s Method. Enzyme activities were measured by using the diagnostic strips and were read on colorimeter [11].
Tissue biochemical assay
Lipid peroxidation was determined by measuring Malondialdehyde (MDA) by Okhawa’s method glutathione was measured by its reaction with 5,5-dithiobis (2-nitrobenzoic acid) DTNB by Ellman’s method. Catalase by Aebi’s method and Superoxide Dismutase (SOD) was determined by Marklund’s method and the total protein content was measured by Lowry’s method.
Histopathological observation
The liver was dissected out and fixed in 10% formalin. Sections were prepared and then stained with haematoxylin and eosin dye for photomicroscopic observation including cell necrosis, fatty change, hyaline regeneration, ballooning degeneration.
Statistical analysis
The data are expressed as mean ± standard error of mean (mean ± SEM). The difference among means has been analyzed by one way ANOVA. The results of all the extracts including standard drug are compared with the result produced by positive control group. A value of p˂0.05 was considered as statistically significant.
Phytochemical screening
Phytochemical screening showed that the ethanolic extract contains poly phenolic compounds, tannins, flavonoids, alkaloids and saponins.
Acute toxicity study
Acute toxicity study showed that the ethanol extract was safe at 2000 mg/kg body weight. Animals were alive, active and healthy during the observation period. Hence 1/10th and 1/5th dose on (200 mg/kg and 400 mg/kg) of the drug was selected for the activity.
Blood biochemical estimation
The detailed results of blood biochemical estimation are presented in Table 1. Administration of PCM induced significant increase in the enzymatic activities of SGOT, SGPT, ACP, ALP and Creatinine (P˂0.05) as compared to normal control group. Oral administration of extract at different doses (200 mg/kg and 400 mg/kg) and silymarin (100 mg/kg) significantly (P<0.05, P˂0.01, P<0.001) decreased elevated levels of serum enzymes to its normal compared to PCM treated rats in both preventive and curative studies.
| Treatment | SGOT IU/L | SGPT IU/L | ACP KA units | ALP | Creatinine mg/dl |
|---|---|---|---|---|---|
| Normal control | 90.7 ± 14.111 | 43.3 ± 8.819 | 1.63 ± 0.2522 | 4.89 ± 0.3933 | 1.20 ± 0.2309 |
| Paracetamol 3 g/kg | 405.3 ± 66.826 | 293.3 ± 17.638 | 7.25 ± 1.299 | 15.29 ± 0.3406 | 5.07 ± 0.811 |
| Standard Silymarin 100 mg/kg | 165.3 ± 37.333 | 66.7 ± 20.276 | 2.41 ± 0.2205 | 5.09 ± 0.7091 | 1.6 ± 0.2309 |
| Allophyluscobbe 200 mg/kg | 250.7 ± 46.495 | 166.3 ± 55.167 | 5.33 ± 0.0833 | 11.17 ± 1.225 | 2.4 ± 0.2309 |
| Allophyluscobbe 200 mg/kg | 213.3 ± 28.221 | 130.00 ± 11.547 | 4.00 ± 0.3819 | 7.64 ± 0.6784 | 1.87 ± 0.4807 |
Table 1: Effect of EEAC on blood biochemical parameters (SGOT, SGPT, ACP, ALP and Creatinine) in control and experimental groups of animals.
Tissue biochemical estimations
Different biochemical estimations were performed in liver tissues which are presented. A significant increase was observed in the level of LPO in the liver when compared with control group (P<0.05). Treatment with different doses of the extract and the standard drug silymarin inhibited the LPO in dose dependant manner and reversed the oxidative stress towards the normal control (P<0.05). GSH, CAT and SOD are supposed to be an important endogenous defense against peroxidative destruction of cellular membrane [12]. In the present study of the administration of PCM significantly decreased the levels of GSH, SOD and CAT (P<0.05). Treatment with the different doses of extract and standard silymarin effectively restored the levels of GSH, SOD and CAT (P<0.05) towards the normal control (Table 2). All two doses of extract improved GSH, SOD and CAT levels in the liver; however, therapy at 400 mg/kg was more effective. Total protein level was significantly decreased by PCM intoxicated animals that was restored towards normal by the doses of extract and standard silymarin significantly; however, maximum restoration was observed at 400 mg/kg (P<0.05).
| Treatment | SODU/ min/ g protein | CATU/mg of protein | GSH nmol/ min /mg protein |  LPO moles MDA/ mg | Total proteins µg/ml |
|---|---|---|---|---|---|
| Normal control | 285.71 ± 7.140 | 4.372 ± 0.0496 | 0.4632 ± 0.0347 | 30.96 ± 3.491 | 188.74 ± 7.140 |
| PCM 3 g/kg | 64.28 ± 12.373 | 1.319 ± 0.328 | 0.1298 ± 0.016 | 178.05 ± 9.82 | 32.76 ± 8.152 |
| Standard Silymarin 100 mg/kg | 242.85 ± 7.143 | 3.586 ± 0.0797 | 0.3357 ± 0.0301 | 81.28 ± 23.041 | 167.15 ± 9.35 |
| Allophylus cobbe 200 mg/kg | 157.13± 68.14 | 2.139 ± 0.0986 | 0.2622 ± 0.0171 | 116.12 ± 10.991 | 80.79 ± 6.811 |
| Allophylus cobbe 400 mg/kg | 221.42 ± 7.143 | 2.618 ± 0.2079 | 0.2989 ± 0.0106 | 97.73 ± 5.121 | 142.86 ± 16.853 |
Table 2: Effect of EEAC on tissue biochemical estimations (SOD, CAT, GSH, LPO and Total proteins) in control and experimental group of animals.
The mechanism of hepatoprotection by the EEAC might be due to its antioxidant potentials, it concludes that the extract reduces the ROS that may decrease the oxidative stress on liver cells and increases the functioning of liver antioxidant enzymes thus prevent the liver from the toxic effects of PCM. EEAC decreased the lipid peroxidation in PCM treated animals and is concluded by decrease in MDA levels.
Histopathological observation
Liver sections of the normal animal showed normal hepatic cells with conserved cytoplasm, prominent nucleus and nucleolus and central vein (Figure 1). Severe degree of liver damage, congestion and macro and micro- vesicular steatosis was observed in liver sections PCM treated animal. Liver section of Silymarin (100 mg/kg) treated animals showed mild degree of liver damage, sinusoidal congestion, mild inflammation and mild degree of macro and micro- vesicular steatosis.
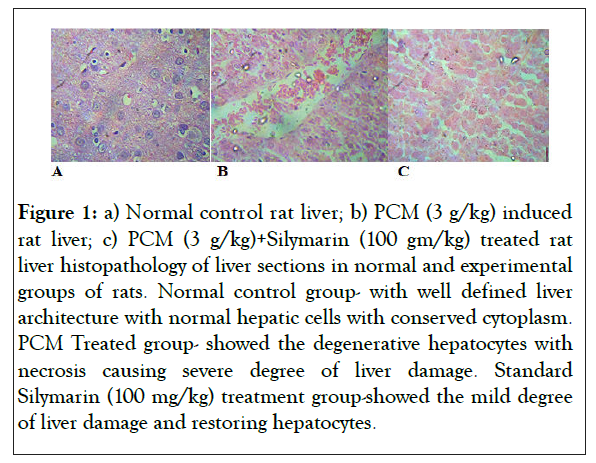
Figure 1: a) Normal control rat liver; b) PCM (3 g/kg) induced rat liver; c) PCM (3 g/kg)+Silymarin (100 gm/kg) treated rat liver histopathology of liver sections in normal and experimental groups of rats. Normal control group- with well defined liver architecture with normal hepatic cells with conserved cytoplasm. PCM Treated group- showed the degenerative hepatocytes with necrosis causing severe degree of liver damage. Standard Silymarin (100 mg/kg) treatment group-showed the mild degree of liver damage and restoring hepatocytes.
PCM at therapeutic doses is most preferred analgesic and antipyretic substance, but when the therapeutic dose exceeds it can produce hepatic necrosis in experimental animals and humans. Alteration in permeability of the cells affects the transport function of hepatocytes causing the leakage of cellular enzymes into the plasma and is the main sign of the PCM induced hepatic injury. Majority of the metabolic reactions are carried out by the liver therefore it is an essential organ which is affected by various chemicals and toxins and liver injuries by various hepatotoxins are main toxicological problems at present. Herbs catch the attention for treatment of various liver diseases to recur the lack of reliable liver protective [13].
Phytochemical investigation of EEAC showed the presence of poly phenolic compounds, tannins, flavonoids, glycosides, alkaloids and saponins. Previous Phytochemical studies with Allophylus Cobbe showed the presence of several terpenoids, saponins seven compound mixture of terpenoids, alkanes and fatty acids, 1,1-diethoxy ethane, phytol and hexanoic acid. Allophylus Cobbe has wide Ethnomedicinal importance and is used in different biological activities like Antiosteoporotic, Wound healing, anti-inflammatory, Antiulcerogenic, ulcer healing activity, Antidiabetic, Antihypertensive, Antibacterial, Antimalarial, Insecticidal hence it proves its medicinal importance.
It is well known that PCM administration alters the hepatic cell membrane permeability, that causes the introduction of cytochrome or depletion of hepatic glutathione and thereby leakage of several enzymes (SGOT, SGPT, ACP, ALP and creatinine) in serum. Reduction in levels of these enzymes is one of the several mechanisms to combat the hepatotoxicity. Notably, EEAC reduced leakage of enzymes in serum, signifying that they protect the liver cell and also maintained normal liver functioning and further stabilized plasma membrane as well as regeneration of damaged liver cells by restoring the hepatic parenchymal cells and normalizing the altered cell permeability.
Antioxidant protective enzymes such as SOD, CAT and GSH are vital in protecting organisms from oxidative damages. SOD converts superoxide radicals to hydrogen peroxide while CAT present in peroxisomes of eukaryotic cells converts hydrogen peroxide to water and oxygen. GSH is the main intracellular nonprotein sulfhydryl containing compound and one of the essential endogenous antioxidants necessary to maintain cellular proteins and lipids in their functional states. Hepatotoxicity characterized by conjugation of excess of NAPQI and GSH diminish the GSH stores and results in formation of GSSG (Glutathione oxidized). This further induces the toxic effects of oxidative stress and remarkably damage the membrane and cells. In the present study, EEAC showed an increase in the concentration of GSH which maintained the cellular proteins and lipids in their functional states; it also increases the levels such as SOD and CAT whose concentration was decreased by the liver injury induced by PCM [14].
Lipid peroxidation is induced by oxidative stress on cellmembrane lipids [15,16]. Toxic products of lipid peroxidation cause extensive damage of macromolecules. Product of lipid peroxidation Malondialdehyde (MDA) determination is commonly used to evaluate lipid peroxidation in animal tissues. EEAC decreased the lipid peroxidation in PCM treated animals and is concluded by decrease in MDA levels.
The mechanism of hepatoprotection by the EEAC might be due to its antioxidant potentials, it concludes that the extract reduces the ROS that may decrease the oxidative stress on liver cells and increases the functioning of liver antioxidant enzymes thus prevent the liver from the toxic effects of PCM . As they are regeneration of the hepatocytes by the improved synthesis of the protein can also be the probable mechanism for the hepatoprotective activity.
The above study revealed that PCM induced liver damage in albino wistar rats, was significantly decreased after being treated with the EEAC leaves, which suggested its hepatoprotective activity.
In conclusion the present study reveals that the Ethanolic extract of Allophylus cobbe possesses strong hepatoprotective activity as it showed protective effect against paracetamol induced hepatotoxicity in rats confirmed by significant decrease in SGOT, SGPT, ACP, ALP, Creatinine, LPO and significant increase in total proteins and protective enzymes such as SOD, CAT, GSH and restoration of histopathological structure of liver damaged by paracetamol hepatotoxicity.
The authors are grateful to Dr. J. I. Disouza, Principal TKCP Warananagar for providing the library and the internet facility for the research work.
Citation: Chavan S, Dias R, Magdum C (2021) Hepatoprotective Effect of Ethanolic Extracts of Allophylus Cobbe (L.) Raeusch Leaves against Paracetamol Induced Hepatotoxicity in Rats. J Appl Pharm. 13:300.
Received: 23-Jun-2021 Accepted: 07-Jul-2021 Published: 14-Jul-2021 , DOI: 10.35248/1920-4159.21.13.300
Copyright: © 2021 Chavan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.