
Anesthesia & Clinical Research
Open Access
ISSN: 2155-6148

ISSN: 2155-6148
Case Report - (2021)Volume 12, Issue 6
In microgravity the human body applies many adaptations to different organs and areas of the body, first of all on the
cardiovascular system. The major change observed is fluid redistribution to the upper part of the body that induces
an increased preload caused by a peripheral venous system emptying. The increased venous return and the
redistribution of blood flow activate a complex neuroendocrine mechanism which reduces the blood volume.
According to previous data, the heart volume is reduced by 10%-17% and it continues to steadily reduce for the
entire period of time spent in space (weeks or months). The resulting reduced muscle mass of the heart has been
wrongly compared to atrophy observed in patients chronically bedridden, geriatric or disabled but it seems to have a
different pathophysiological substrate. This phenomenon may be explained by the ability to remodel the left ventricle
depending on working conditions; it can reduce its muscle mass as a consequence of hypovolemia and a reduction in
peripheral resistance (baby heart).
In order to clarify hemodynamic and neuroendocrine abnormalities and their basis due to microgravity, where
hemodynamic and metabolic requirements are reduced, we introduce the concept of Baby Heart Syndrome
(BHS).On the contrary, upon their return to earth, astronauts show typical manifestations of heart failure in the
upright posture, due to the re-filling of the venous reservoir in gravity. In order to relieve these symptoms, some
countermeasures are currently used such as the Head down Tilt (HDT) performed before departure and the Lower
Body Negative Pressure (LBNP) in microgravity.
We propose a new measure to improve symptoms during the first days in space and to accelerate the re-adaptation to
Earth's gravity. We suggest the creation of a Blood Bank Space in Space (BBSS) for astronauts, which involves a blood
sample once they reach the microgravity. This procedure would reduce the volume increase of the heart, and at the
same time would constitute a useful reserve of blood to re-infuse to astronauts on their return to the earth, thus
reducing orthostatic intolerance. The possibility to re-infuse autologous blood could also remove infective or
transfusional risk.
Anesthesiology; Sedation; Microgravity; Local anesthesia; General anesthesia; Surgical anesthesia; Baby heart syndrome; Hypovolemia; Vascular anesthesia
In microgravity the human body applies many adaptations to different organs and areas of the body, first of all on the cardiovascular system. The first major adaptation observed is the redistribution of fluids in the upper part of the body [1,2]. In normal conditions, the venous system contains about 64% of the blood volume (2500-3500 ml) and its pressure varies depending on the position of the body in the Earth’s gravitational system (Figure 1).
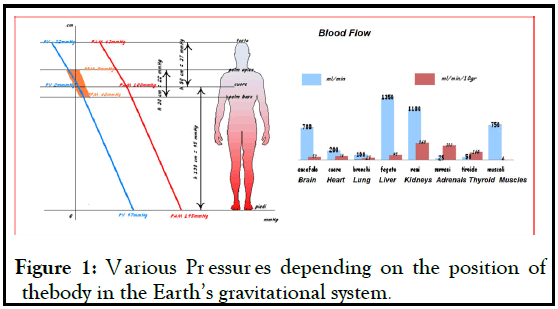
Figure 1: Various Pressures depending on the position of thebody in the Earth’s gravitational system.
In microgravity, blood flow tends to return to the heart more easily because of the lack of hydrostatic pressure effect in the lower areas of the body; in addition, the difference in arteriovenous pressure appears the to be the same in all regions of the body. In microgravity, this determines a redistribution of this large portion of blood contained in the venous district below the heart line, in favour of high venous or arterial compliance organs, such as liver, spleen and lungs. The redistribution of blood according to the new needs of the organism causes also the resulting increase in venous return to the heart which triggers a complex neuroendocrine mechanism to change the blood volume.
Studies conducted during short spaceflights or in experimental models like the Head down Tilt (HDT) observed, after a few hours of stationing in weightlessness, a significant reduction in blood volume (10%-17%), a reduction by 25% in stroke volume, myocardial tissue, and cardiac mitochondrial enzymes in muscle strength of 23%-50% [3].
The redistribution of blood flow coming from the lower districts results in an increase of the atrial filling pressure [4]. During the first 24 hours at 0G a dilatation of the cardiac chambers by about 20% and an increase of the secretion of Atrial Natriuretic Peptide (ANP) up to 80%, compared to normal values, were observed [5]. Thanks to the activation of several neuroendocrine mechanisms these adaptations lead to the reduction of the renal reabsorption of sodium and water with consequent hypovolemia and reduced pressure rate. Therefore, the organism is in a condition similar to an excessive blood transfusion, which also determines a reduction of erythropoietin in response to the excess of blood volume [6].
Concerning the lungs, at 1G pulmonary circulation pressures are particularly low and pulmonary arteries are easily distensible with the increase in blood volume (Figure 2) while , in microgravity, the blood distributed in the pulmonary vessels no longer meets the normal areas of West [7].
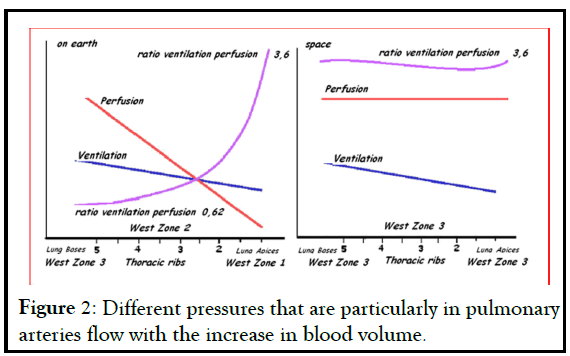
Figure 2: Different pressures that are particularly in pulmonary arteries flow with the increase in blood volume.
In fact, in every area of the lung a dilation of vessels with the increase in pressure can be observed that may also exceed the values of alveolar pressure. It follows a recruitment of those vessels which normally are closed, a reduction in pulmonary vascular resistance and an increase in lung diffusion capacity [8]. The improvement of alveolar gas exchange observed during the early days would be related to the redistribution of blood flow that makes an improvement in pulmonary diffusion capacity possible. However, the improvements regress to the pre-flight conditions in long missions. There is no supporting data, but it is possible that after the first days a subclinical interstitial edema could appear and decrease the oxygen diffusion, returning to the partial pressures of arterial O2 values found on earth.
If compared to the modifications studied on HDT simulation models, the mechanism of fluids regulation in space is different in several aspects: the increased diuresis due to ANP observed in the simulation HDT does not occur in space flights, where a reduced diuretic and natriuretic response to saline intake was observed [9,10]. In addition, in space a lowering in the plasma volume, hematocrit and plasma protein concentration was observed. The central venous pressures remain unchanged as well as the mean systemic pressure and heart rate. The increase in cardiac output that persists for approximately one week by stationing in space is due to vasodilatation [11]. Mediated by the sympathetic nervous system and the renin-angiotensin [4]. While the increase in the cardiac volume is the consequence of the initial increase in atrial filling pressures for the increase in venous return by 25% [12]. The cardiovascular changes observed, however, disappear after one week spent in microgravity [13].
Martin et al. noted that after 129 and 144 days spent in space, the ejection fraction of the left ventricle is reduced [6,14]. Had previously concluded that after 8 months in space, there was no impairment of myocardial contractility. In 2005, Summers et al. have documented through an ultra sound study a reduction in muscle mass of the right ventricle by 9% [15]. Concluding that mass loss of the left ventricle would not be due to muscle atrophy, but to the variation of blood volume; back on earth the original heart volume would be restored in just three days. A ventricular dilatation follows the increased blood flow to the left atrial level.Studies on changes in blood pressure, heart rate and ejection fraction during parabolic flight defined the adaptation to microgravity that occurs in a short time [16]. Cardiac mass is reduced after the first 24-48 hours in space, and remains stably reduced throughout the entire period spent in vol. This appears to be due to the ability of the cardiac musculature to adapt to the work demands of the left ventricle, increasing or reducing its own muscle mass for the work required by the increase or reduction in blood mass.
The physiological remodeling of cardiac muscle in adaptation to microgravity has been incorrectly compared to what has been observed in patients chronically bedridden, geriatric or disabled. However astronauts in microgravity have healthy hearts, which have to reduce their work because of a decrease in metabolic organs demand, and that for hemodynamic conditions is more similar to a “baby heart”. These changes would allow an energy saving and a consequent centralization of the bloodstream due to a reduced capacity of peripheral venous no longer supported by the gravitational force and to a reduction in overall metabolic body needs (Figure 3).
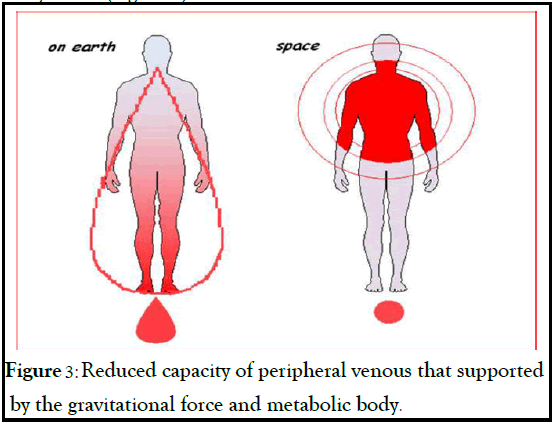
Figure 3: Reduced capacity of peripheral venous that supported by the gravitational force and metabolic body.
One of the key features of the Baby Heart Syndrome is the reversibility. The syndrome affects the astronaut who remains for extended periods in space through the hemodynamic changes that are perfectly compatible with life in microgravity. The occurring adaptations, however, cause many problems by returning to earth where, due to gravity, a large blood volume is required to replace the venous reservoir that had been emptied in space. For this reason the finding of orthostatic intolerance in astronauts returning from space mission is frequent.
This condition is similar to that observed in a syncope bleeding patient who has lost a substantial amount of blood, loss that does not allow the patient to stand in the upright position for hypotension; it is closely related to the body’s need for a greater blood volume, due to the theft of blood in the venous district from the lower limbs.
The introduction of the Baby Heart Syndrome concept is therefore important to understand the regulation of cardiac function at minimum levels, reversible after re-adaptation to gravity on earth. The speed of the symptoms regression depends on the recovery speed of the blood volume lost in microgravity, necessary for the metabolism of various organs.
The best understanding of the Baby Heart Syndrome leads to the hypothesis of new countermeasures that can be taken instead of or in addition to those which are already used, like HDT and Lower Body Negative Pressure (LBNP) [17].
The aforementioned mechanisms are activated for the sole purpose of reducing the heart workload by reducing the blood volume because it is so requested by the other organs and systems. One of the currently used methods to counteract the shift of blood volume between the different body compartments aims to keep the astronaut in Trendelemburg position of at least 6 degrees (- 6°C HDT) for several days before space flight. This method allows to control all the effects of hemodynamic changes on the grounds and to be able to intervene immediately in case of need, which is impossible in space.
But in order to compensate for the decrease in plasma volume that occurs after exposure to microgravity and the anemia resulting from all the phenomena that require the involvement of all other organs (brain, lungs, kidneys, bones and muscles), we suggest the creation of a "Blood Bank Space in Space" BBSS (Blood Bank Space in Space) for each astronaut which must work in microgravity conditions. The concept of a “blood bank” would involve the removal of a blood share as soon as the astronaut reaches the microgravity conditions; the collected blood would then pumped into an artificial reservoir outside the body (BBSS store).
The proposed countermeasure would thus have a twofold advantage. On the one hand, the removal of a blood share would reduce, shortly after the arrival in microgravity, the atrial distension, the ANP release and the neuroendocrine and biochemical alterations due to the redistribution of blood volume (Figure 4).
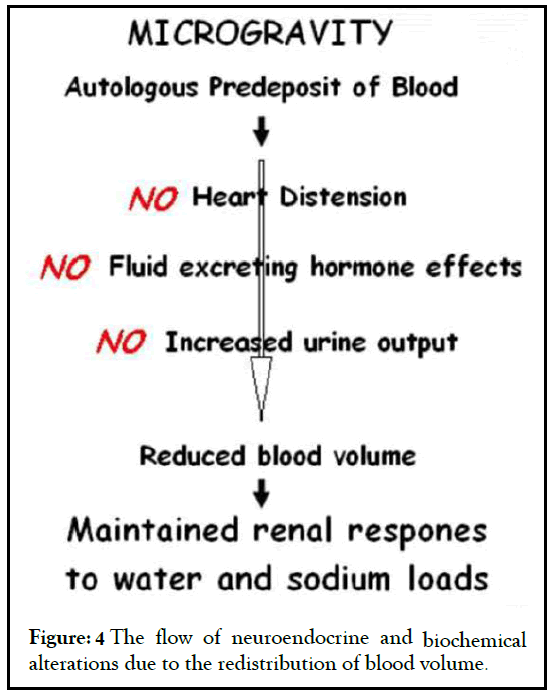
Figure 4: The flow of neuroendocrine and biochemical alterations due to the redistribution of blood volume.
On the other hand, the collected blood, if properly stored, can be an essential support on returning to the earth. In fact, it could represent a useful blood reserve to re-infuse in order to prevent the typical orthostatic intolerance symptoms due to the blood volume reduction on returning to the earth. So, the BBSS might also facilitate the astronaut’s re-adaptation by his returning to earth.Current systems used to make blood deposits for elective surgery have proven effective in sparing homologous blood transfusion and in reducing infection risks. The same concept can be applied to astronauts who must stay in space for a long time; their risk can be compared to the risk of blood loss during an operation, which is expected and supported.
With modern techniques of conservation between +2 and +6°C, blood bags can be infused from 36 to 42 days after collection. In case of long space flights, the blood stored in the BBSS system can be kept fresh using the "Jumping Frog” technique, which involves removing fresh blood and re- infuse blood bags near expiration dates (within 36-42 days).
The problems of blood taking and infusion in microgravity can be solved by means of syringe pumps as an alternative to blood bags that require the gravitational system for the infusion with the infusion tube. The astronauts could avoid the preparation in the position -6°C HDT, but they should place a tunneled venous catheter to be removed only after the restoration of blood volume contained in the BBSS on returning to earth. The venous catheter should be used in space for blood sampling and infusion, in order to spare venous punctures and to avoid infection risks. Furthermore, the catheter may be used to perform blood tests in space, which was impossible until today. No changes to existing countermeasures would be needed. Indeed, the procedures laid down like LBNP could be a valuable aid to maintain constant stimulation of bone marrow function and to train the body to relative hypovolemia that occurs on returning to the earth.
New hypothesis on the effects of weightlessness in human body pushing to evaluate new strategies and countermeasures that can allow the stationing for a long time in space, without causing irreversible alterations. The BHS is due to the changes that the organism activates to reduce unnecessary blood volume in microgravity. On his return to earth, an astronaut behaves like a bleeding patient who lost in a few hours up to 64% of blood volume seized in the peripheral venous district.
The BBSS we suggest can be used to prevent the adaptation to microgravity symptoms and to aid the organism re-adaptation at return to earth. Thanks to new technologies, the blood collected is able to maintain the same stored both in weightlessness and on the earth. Although experimental studies are needed to confirm this theory, the possibility of relieving the symptoms related to microgravity and reinfusing autologus blood without infective of transfusional risks could be an important advantage for the immediate recovery of the physical ability of the astronauts.
Citation: Passarani S (2021) Hemodynamic Changes of Microgravity and Adaptation In Gravity: "Baby Heart Syndrome (BHS)" and Evaluation of New Countermeasures of Microgravity in the Human Body. J Anesth Clin Res. 12:1012.
Received: 04-Jun-2021 Accepted: 18-Jun-2021 Published: 25-Jun-2021 , DOI: 10.35248/2155-6148.21.12.1012
Copyright: © 2021 Passarani S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.