
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2022)Volume 12, Issue 6
The emergence of immunotherapy has caused a wave of co-development of anticancer drugs globally, and some unreasonable or unwarranted combination of anticancer drugs was observed. Combining regulatory guidance with case studies, this study aims to clarify key generally applicable principles in the co-development of combination therapy, when it’s appropriate to start a confirmatory trial, possible scenarios and main determinants of the most efficient confirmatory trial design. Biological rationale for the combination, adequate safety profile of each individual drug and the combination, added efficacy of the combination and attribution of each individual drug, are three golden basic principles for anticancer combination therapy. Before initiation of a confirmatory trial, a powered factorial study supporting the superiority of the combination over individual drug and standard-of-care is recommended. The appropriate design of pivotal study is generally a class-by-class determination, mainly based on whether there is a similar drug approved for the more active drug, what has been previously demonstrated about the effects of the combination and the individual drugs, the feasibility of monotherapy and standard-of-care alone treatment arms, the best practice and other factors.
Neoplasms; Combination therapy; Statistic design; Confirmatory trial
Cancer is a complex disease that represents one of the leading causes of death in many countries. In addition to genetic diversity, cancer cells in a tumor can be heterogeneous and various, resulting in different responses to one certain drug [1,2]. Combination therapy generally refers to the co-administration of two or more therapeutic agents, can generate synergistic anticancer effects by focusing on different signaling pathways in tumor cells, thus could overcome mechanisms of resistance and minimize side effects. For example, combining checkpoint inhibitors with standard-of-care chemotherapy has been successful in nonsmall cell lung carcinoma and small cell lung cancer. With the clinical success of immune checkpoint inhibitors over the past 10 years, the emergence of immunotherapy has stimulated a wave of co-development of anticancer drugs globally [3-6]. Investigators and pharmaceutical companies were inspired to explore the potential of the co-development of various combination therapies in different cancer types and stages [7].
To assist sponsors in the co-development of two or more new investigational drugs, in 2013, the US Food and Drug Administration (FDA) issued the first guidance [8]. However, the guidance mainly describes high-level regulatory issues and generally principles, specific to novel-novel combination, which means novel drugs that have not been previously developed for any indication of the targeted disease globally. In reality, many combinations involved at least one previously globally approved drug. Consequently, the standards or requirements in FDA guideline are not applicable for the situation.
By the end of 2020, National Medical Products Administration (NMPA) of China issued the guideline for the co-development of combination therapy specific to anticancer drugs, with an emphasis on principles of trial design and benefit evaluation [9]. This guidance included combinations of two or more investigational drugs, an investigational drug with a previously approved drug for a different indication, or two (or more) previously approved drugs for a different indication as a novel combination therapy. Unfortunately, the dissemination and understanding of the guidance were largely limited as it was in Chinese and lack of case example, which can indicate critical considerations for different scenarios and corresponding appropriate design for pivotal studies.
Previous review demonstrated that 72% trials lacked significant preclinical evidence supporting the development of the anticancer combination in the given indication [7]. In our review of proposed projects, some unreasonable or unwarranted combination of anticancer drugs was also observed. For example, the combination development is carried out in the absence of sufficient safety data of the individual drugs or primary efficacy data in targeted population, or the efficacy of the combination is only compared to SoC (standard of care), lacking of efficacy evidence on single component’s contribution. In addition, the setting of appropriate control group in the confirmatory trials as well as its rationale is still a critical problem troubling physicians and researchers. Such as, why a randomized two-arm trial comparing the combination with SoC is acceptable for IMBARVE 150, while a randomized three-arm trial is needed for HIMALAYA study, given both combination therapy targets on the first line therapy for liver cancer [10,11]?
Considering the actual existing problems and complexity of the development of combination therapies in oncology, it can be essential to clarify key generally applicable principles in the codevelopment of combination therapy, when it’s appropriate to start a confirmatory trial, possible scenarios and main determinants of the most efficient confirmatory trial design. Therefore, this study is intended to help the reader to answer the above questions, combining regulatory guidance with case studies, and thus facilitate the scientific and orderly codevelopment of effective combination therapy.
General principles applicable for exploratory trial design of the co-development of anticancer combination therapy
Using two or more drugs together as treatment is a simple idea with a complicated underpinning. Assuming we have 10 available A drugs, 10 available B drugs and 10 potential targeted populations, you’re making a three-way combination, then there are more than a thousand combinations. It’s hard to know what drugs and doses will work together in which population, because drugs in combination don’t always act together the way you expect them to base on how the drugs perform alone. In addition to biological rationale for the combination, sponsors should develop evidence on potential added effect in the preclinical study or exploratory trial of the combination, or from reported trial data of a similar combination pattern [12].
Generally, co-development will provide less information about the clinical safety, dose-response and effectiveness of the individual new investigational drugs intended to be used in combination than would be obtained if they were developed alone. Therefore, the safety profile of each individual new investigational drug should be characterized in phase I studies in the same manner as would be done for the development of a single drug whenever possible, including determination of the Maximum Tolerated Dose (MTD), the nature of the Dose Limiting Toxicity (DLT), and pharmacokinetic parameters [8].
Sufficient monotherapy safety profile should be depicted prior to initiation of combination therapy. And careful combination dose escalation, which is generally recommended to start from one dose under phase II dose (RP2D) of the monotherapy, should be performed to find the RP2D of the combination, considering the synergetic effect of two or more components [8,9]. It may be also acceptable to conduct the combination dose escalation directly from the RP2D of one or more single agents, which is context-dependent and specific to the drug, previous evidence from similar combination, indication, and need of the patient population at the time of development [13].
Randomized factorial design: An efficient strategy for demonstrating early activity and attribution for combination therapy
In order to demonstrate the superiority of combination therapy versus control drug, and to show the contribution of each component, a randomized factorial design is recommended in phase II trials when necessary [14]. Figure 1 shows the classical design of 2*2 factorial study, allowing us to simultaneously look for the combined effect, the attribution of each component, interactions between drug A and drug B. The combination is addictive when the combined effect is greater than the larger effect of each individual drug, and the combination is synergistic when the combined effect is greater than the additive effect of each individual drug. Both addictive and synergistic effect is considered as with added efficacy.
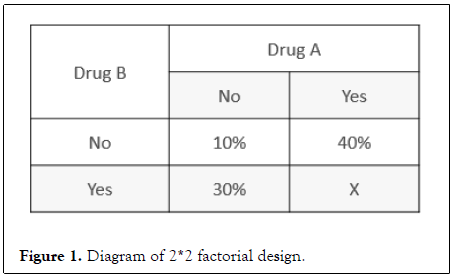
Figure 1: Diagram of 2*2 factorial design.
Here, group received neither drug A nor drug B is deemed as SoC. The attribution of the combination mainly depends on the direct comparison of clinical efficacy in the two monotherapy arms with the SoC arm and influences the necessity of one or more monotherapy arm. If the results of factorial analysis suggest that efficacy of all monotherapies is worse than SoC statistically, then it becomes no need to set the monotherapy arm in confirmatory trials. If the more active drug according to independent effect, assumed to be drug A, is suggested comparable to SoC, then the A monotherapy arm is required in confirmatory trials. Another situation, A monotherapy is demonstrated to be better than SoC with statistical significance or the magnitude of effect is large enough, then the SoC arm becomes unnecessary.
There may be situations where one component or partial components from the combination therapy maybe inefficacious when work alone, such as 1) biomarker driven or associated activity, 2) rapid resistance to an individual agent, 3) the combination is in a disease setting where there is no or very little single agent activity. In these situations, a factorial design would be unnecessary. Regarding to acceptable endpoints in early factorial trials evaluating two or more drugs for use in combination, it’s also context-dependent. Intermediate endpoints including Progress-Free Survival (PFS) and Objective Response Rate (ORR) are frequently used.
Critical criteria determining the timing for conducting confirmatory trials of the codevelopment of anticancer combination therapy
We should also be fully aware that combination therapy will possibly increase the safety risk of participants, and it may be a waste of resources to conduct large sample size confirmatory trials without sufficient evidence to demonstrate add-on efficacy for combination therapy compared with each component as monotherapy. It’s highly recommended to ensure the following criteria before the formal implementation of confirmatory trials on the co-development of anticancer drugs for use in combination: 1) scheduled dosage and timing for the combination was optimized; 2) compared with SoC, potential added efficacy with acceptable toxicity, supporting the combination, was observed; 3) attribution of each individual drug in the combination was demonstrated to the extent needed. In the case of uncertainty about the attribution of individual drugs, an adaptive confirmatory trial design with multi-arm might also be used, terminating the single drug arms early if it becomes clear that the single agents have much less activity than the combination.
Possible scenarios and main determinants of confirmatory trial design in the co-development of anticancer drugs for use in combination
The possible scenarios and appropriate design of phase III trials could be generally classified into 6 scenarios of 2 types (Figure 2), according to conditions if there is a drug A’ that has the same mechanism of action and target with drug A approved, clinical efficacy of A monotherapy and the best practice in targeted indication, and other factors. The following scenarios illustrate possible phase III study designs for combinations of two new investigational drugs in different situations, followed by a case example, respectively. The former three scenarios illustrated the circumstances that there isn’t any drug A’ approved.
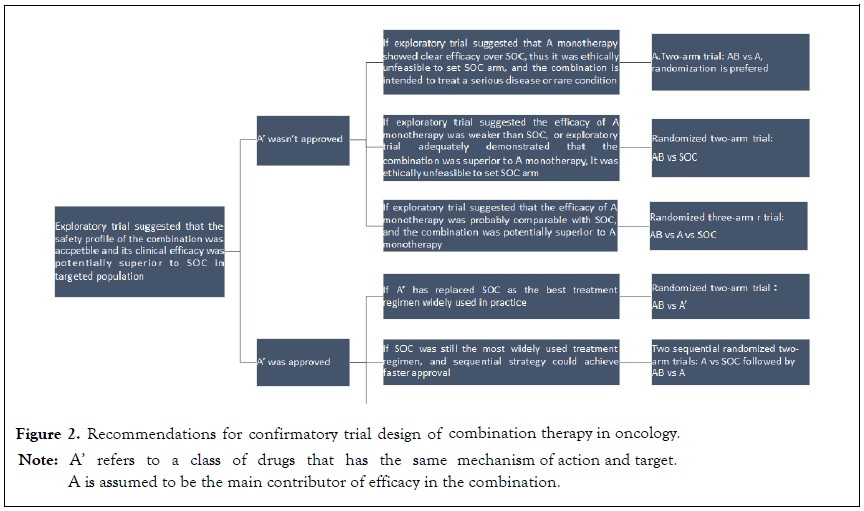
Figure 2: Recommendations for confirmatory trial design of combination therapy in oncology.
Note: A’ refers to a class of drugs that has the same
or the magnitude of effect is large enough, then the SoC arm
becomes unnecessary.
mechanism of action and target.
A is assumed to be the main contributor of efficacy in the combination.
Scenario 1: The efficacy of A monotherapy has been suggested to be superior to SoC that it’s ethically unfeasible to set SoC arm, and the combination is intended to treat a serious disease or rare condition with high unmet medical needs, then a twoarm trial comparing AB with A is recommended, with a randomized trial preferred. The result that AB combination is significantly better than A monotherapy, and A monotherapy is better than SoC, if existed, is expected by regulatory agency to grant the approval of the combination. The evidence on superiority of A monotherapy to SoC could be concluded from previous study on targeted population, including randomized factorial trials, non-randomized trials or even Real-World Study (RWS). Additionally, if tested drug is biomarker driven, the evidence could also be derived from definite efficacy improvement in other indications. BRF113928 (NCT01336634) was the pivotal study supporting the regular approval of dabrafenib and trametinib combination, for metastatic nonsmall cell lung cancer (NSCLC) with BRAF V600E mutation in 2017, which is observed in 1% to 2% of lung adenocarcinomas and act as a rare oncogenic driver and poorer prognostic factor in NSCLC [15]. In melanoma patients with BRAF V600E mutation, it had been demonstrated that dabrafenib was superior to best supportive care and the combined regimen showed addictive effect in 2015 [16]. A nonrandomized open-label three-cohort trial was adopted for two indications, including dabrafenib monotherapy for previously treated BRAF V600E mutation NSCLC, dabrafenib plus trametinib combination for previously treated BRAF V600E mutation NSCLC, and dabrafenib plus trametinib combination for naïve BRAF V600E mutation NSCLC [17-19].
Scenario 2: SoC is more efficacious than both of the component drugs, or the combination has been demonstrated to be superior to A monotherapy, that it’s ethically unfeasible to set monotherapy arm, then a randomized two-arm trial comparing AB with SoC is recommended. Evidence showing the superiority of AB combination to SoC is expected by regulatory agency.
The combination of atezolizumab plus bevacizumab showed encouraging antitumor activity and safety in a randomized cohort of phase 1b trial involving patients with unresectable Hepatocellular Carcinoma (HCC), with the ORR of 36% and a median progression-free survival (PFS) of 7 months, and the hazard ratio for PFS compared with the more active bevacizumab was 0.55 (0.40, 0.74), which suggested the superiority of the combination and the contrary to ethics to set the monotherapy arm in confirmatory trial [20]. Several confirmatory trials have shown inadequate activity of single immunotherapy drug as first and second line treatment in HCC patients, such as Checkmate 459 and Keynote 240 [21,22]. Therefore, in its pivotal trial known as IMBRAVE 150 (NCT03434379), a case of two previously approved drugs for a different indication as a novel combination therapy, a randomized two-arm trial (AB vs. SoC) was accepted [10].
In contrast, in the design of the combination of tremelimumab plus durvalumab for unresectable HCC patients known as HIMALAYA study (NCT05345678), a more costly confirmatory design of randomized three-arm trial (AB vs. A vs. SoC) was required [11]. Because, according to its exploratory trial, combined effect has not been demonstrated to be superior to A monotherapy, and the monotherapy is potentially comparable with SoC (Scenario 3) [23].
In case of drug A’ approved in targeted population, the setting of the control arm should give full consideration of the best treatment at present, which is generally the most effective regimen widely used. There are three detailed scenarios involved, that is the following Scenarios 4-6. If A’ has replaced previous SoC as the up-to-date SoC, then a randomized two-arm trial (AB vs A’) is acceptable as pivotal study (Scenario 4). For example, at the initiation of MARIPOSA study (NCT04487080) in 2020, the confirmatory trial of two investigational drugs, lazertinib plus amivantamab combination in locally advanced or metastatic NSCLC, osimertinib, the EGFR tyrosine kinase inhibitors, which has the same mechanism of action and target with lazertinib, has been widely used as the best treatment in NSCLC. Hence, the primary endpoint of MARIPOSA study is the combination will demonstrate superior PFS compared with osimertinib [24].
If drug A‘hasn’t been widely used due to cost or other issues which cannot replace the current SoC, then we should prove to the regulators that a given combination therapy is superior to A monotherapy, and the monotherapy is superior to SoC. In this case, a sequential strategy (two sequential randomized two-arm trials, Scenario 5) or synchronous strategy (one randomized three-arm trial, Scenario 6) could be adopted, mainly depending on whether the medication of the combination has been explored.
Supposing the dosage or timing of the combination hasn’t determined while that of the monotherapy has been determined, we could achieve faster approval of the monotherapy by carrying out a randomized two-arm trial (A vs. SoC), and a subsequent randomized two-arm trial (AB vs A) could be implemented when appropriate combination regime is determined. The co-development of tiragolumab plus atezolizumab combination, an investigational drug with a previously approved drug for a different indication, in PD-L1 selected patients with treatment-naïve NSCLC adopted the sequential strategy that atezolizumab was approved firstly in 2018 and the combination was subsequently studied in SKYSCRAPER-01 study (NCT04294810) [25,26]. If this is not the case, it’s commonly endorsed to conduct a randomized three-arm trial (AB vs. A vs. SoC), as it gains higher statistical power under the same sample size.
Biological rationale for the combination, adequate safety profile of each individual drug and the combination, added efficacy of the combination and attribution of each individual drug, are three golden basic principles for in the early phase development of anticancer combination therapy. Experiences from sponsors also demonstrated that findings in animal models are not easily translated to clinical predictions, indicating a need for better pre-clinical models. Even it’s not easy to translate the results from single-arm trials into prediction of phase III benefit. Randomized trials, especially factorial studies, were thus acknowledged.
Additionally, as added efficacy being critical in early development, the magnitude and significance of effect required should be communicated with regulators in advance. A powered factorial study and statistical analysis supporting the superiority of the combination over individual drug and SoC before initiation of a confirmatory trial, using surrogate endpoint is favored by regulatory agency. Trial sponsors and regulators should also balance the level of evidence needed for approval in the context of data that may already be available to ensure equipoise and expedite development. Other regulatory issues, such as investigational new drug application, risk control plan, marketing application should also be clarified.
The appropriate design of pivotal study generally is a class-byclass determination, mainly based on whether there is a similar drug approved for the more active drug in the combination, what has been previously demonstrated about the effects of the combination and the individual new investigational drugs, the feasibility of monotherapy and SoC alone treatment arms, the best practice and other factors. It is important for sponsors pursuing the development of a combination to initiate conversations with the regulatory agency early in their development programs, to determine if they meet the above criteria of conducting a confirmatory trail and, if so, whether they are pursuing the most efficient path forward. Though this study is carried out based on the combination of two new anticancer drugs, the key viewpoints and recommendations are also suitable for the situation of multi-drug combination.
The authors Huiyao Huang, Yu Tang, Dandan Cui, Yinyu Meng and Dawei Wu are contributed equally to the manuscript.
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
Citation: Huang H, Tang Y, Cui D, Meng X, Wu D, Liu M, et al (2022) General Principles and Confirmatory Trial Design for the Co-development of Anticancer Drugs for Use in Combination. J Clin Trials. 12:513
Received: 01-Dec-2022, Manuscript No. JCTR-22-20642; Editor assigned: 05-Dec-2022, Pre QC No. JCTR-22-20642 (PQ); Reviewed: 19-Dec-2022, QC No. JCTR-22-20642; Revised: 23-Dec-2022, Manuscript No. JCTR-22-20642 (R); Accepted: 26-Dec-2022 Published: 30-Dec-2022 , DOI: 10.35248/2167-0870.22.12.513
Copyright: ©2022 Huang H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.