Fisheries and Aquaculture Journal
Open Access
ISSN: 2150-3508
ISSN: 2150-3508
Research Article - (2017) Volume 8, Issue 4
Fecundity and condition factor of Clarias angullaris were studied in Oguta Lake for eight weeks. Forty species of C. anguillaris were studied. The length-weight relationship, fecundity, condition factor and gonad-osomatic indices were factors employed for assessment of health status of the fish. The mean length and weight of the C. anguillaris collected were 27.46 ± 1.47 (12.00-38.50) cm and 391.26 ± 17.92 (144.60-553.40) g respectively. Some of the morphometrics manifested linkage and C. anguillaris exhibited a coefficient of determination (R2) of 0.008, and Pearson’s correlation coefficient (r) of -0.091. Fecundity-weight relationship of C. anguillaris was also not significant (p=0.062) with a Pearson’s correlation coefficient (r) of -0.357 and coefficient of determination (R2)=0.127. The variations in condition factor of C. anguliaris at Oguta Lake was 19.96% (R2=0.1996) and this was explained by the variations in GSI. Clarias anguillaris from the Oguta Lake showed negative allometric growth pattern which implied that the fish species is increasing in length faster than its weight. This could be as a result of the polluted nature of the Lake, shortage of food supply, diseases which might have altered the fish speciation.
Keywords: Clarias anguillaris; Fecundity; Condition factor; Oguta lake
Clariidae fish species are known for its commercial value to people of Oguta. Fish oil contains vitamins A, D, E and K which have been successfully used in controlling coronary heart diseases, arthritis, atherosclerosis, asthma, auto immune deficiency diseases and cancer [1]. Fish is often recommended for cardio-vascular disease patients because of its unique fat, which is composed mainly of Omega-3 polyunsaturated fatty acid. In addition to its nutritious flesh, vitamins A and D present in fish oil are important especially in infants and children [2]. Fish also supplies to the body, a range of inorganic minerals such as Phosphorus, Fluorine, Potassium, Iron, Zinc, Magnesium, and Copper in marine species Iodine as well as vitamins A and B complex [3]. The proximate composition, nutritive values and mineral composition of fishes in Nigeria have been documented [4].
C. anguillaris is a native of Africa and air breathing catfish which is also known as the mudfish. This species is of importance in commercial fisheries. It grows to a length of 100 cm. They are used extensively in aquaculture on account of the ready availability of their seeds in the wild, good adaption to climate ability to support high population densities and to feed on grasses and weeds in the ponds. They feed mainly on fish larvae, molluscs, crustacean and diatoms mostly during the rainy season. Their growth is quite remarkable and pronounced during length-weight relation (LWR).
The condition factor in fish serves as an indicator of physiological state of the fish in relation to its welfare [5] and also provides information when comparing two populations living in certain feeding density, climate and other condition [6]. Thus, condition factor is important in understanding the life cycle of fish species and it contributes to adequate management of these species, hence, maintaining the equilibrium in the ecosystem [7].
The understanding of the biology, environmental parameters and population structure of C. anguillaris is essential to optimize production from the wild. There is paucity or no literature on fecundity and condition factor of C. anguillaris of Oguta Lake. The only known information on Oguta Lake was the ones reported by Okonkwo et al. [8]. A study of fecundity and condition factor of C. angularis species from Oguta Lake will provide information for management decision and culture of the species in the area and similar water bodies. Hence, this work investigated the fecundity of different batch weight of C. angularis species, fecundity-weight, relative fecundity-length relationship, length-weight relationship and relative fecundity-weight relationship. Also the condition factor and gonadosomatic index of C anguillaris in Oguta Lake was as well determined.
Study area
Oguta Lake is one of the inland fresh water drainage basins within South-eastern Nigeria. It lies approximately within Latitudes 50° 41’ and 50° 44’ North and Longitudes 60° 50’ and 60° 45’ East. It is located in Imo State, Nigeria. About linear in shape, the lake receives inputs from Rivers Njaba, Awbana and Orashi, while the fourth river, Utu flows in only during the rainy season The Lake is a relatively small and shallow water body, the water volume increases tremendously during the rains; its maximum surface area being 2.48 Km2 with a depth of 9.30 m [8].
This lake is the largest natural lake in South Eastern Nigeria; it is also a source of navigation and transportation, sightseeing and tourism, and a pool from which to obtain 80% safe and cheap animal protein and other indispensable nutrients for healthy living; thus contributing to the socio-economic development of the zone. Ongoing activities in the Lake include transportation by means of paddle and dugout canoe, or the use of engine-powered boats; peeling, washing and fermentation of cassava (a root crop); others include washing of clothes, cars, motor cycles and kitchen utensils, bathing, disposal of domestic wastes and fishing.
Fish collection
Fish samples were collected from Oguta Lake Figure 1 in Imo state station along the River from artisanal fishermen who used gill nets of mesh sizes ranging from 150 mm-200 mm. Baskets, traps and hook and lines were also being used. Fish collected was transported in a plastic container with water to the Fisheries Research Laboratory, Department of Zoology and Environmental Biology, University of Nigeria, Nsukka for analysis. Sampling was done within 8 am-12 noon twice in a month.
Sex determination
Sex of fish was determined using microscopic examination of the gonads and was determined based on the availability of the gonad. Eggs are readily discernible in the ovary of the mature females while testis is typically smooth, whitish and non-granular in appearance.
Determination of morphometric and biometrics indices
The sampled specimens were examined for total length of the body using a standard meter rule mounted on a dissecting board which was recorded in centimetre, while weight was determined using an electronic balance. The length-weight relationship of the fishes was determined using the power curve:
Where;
W=wet weight (g) of fish;
L=standard length in centimetre;
a and b are regression indices;
Gonado-somatic index estimation: Gonado-somatic index was calculated thus;
GSI = [Gonad Weight/Total Tissue Weight] x 100 [9]
Where;
GSI=gonado-somatic index;
L=left ovary weight (g);
R=right ovary weight (g) and
BW=body weight of fish (g).
Average GSI index was determined.
Fecundity estimation: Fecundity was studied by examining matured ovaries using gravimetric method.
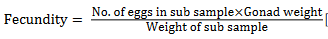 [9]
[9]
Fecundity-length relationship: The standard length will be estimated using the formulae
F=aLb
Where;
F=fecundity;
L=standard length (cm);
a=intercept of the regression with the Y-axis (regression constant) and
b=slope of the regression line (regression constant)
Fecundity-weight relationship: This estimated using the formulae
F=aWb [10]
Where;
F=fecundity;
W=weight (cm);
a=intercept of the regression with the Y-axis (regression constant) and
b=slope of the regression line (regression constant)
Condition factor: The condition factor (K) was calculated as:
Where, W=weight of fish (g), L=Length of fish (cm) [10].
Data from this experiment was computed using Microsoft Excel and exported to SPSS (Statistical Package for Social Sciences) version 20.0 for statistical analysis. The data were subjected to one way analysis of variance (ANOVA) using Duncan multiple range test to compare the mean fecundities for the weights. Relationship between total length and body weight, total length and fecundity, body weight and gonadosomatic index, body weight and fecundity were determined using Pearson’s correlation.
Descriptive statistics of C. anguillaris from Oguta Lake
The mean values of C. anguillaris sampled at Oguta Lake were 27.46 ± 1.47 (12.00-38.50) cm and 391.26 ± 17.92 (144.60-553.40) g respectively (Table 1). The mean fecundity and mean relative fecundity were 14892.31 ± 2854.20 (3257.14-76377.36) and 49.82 ± 9.87 (6.27-21.85) respectively. Also the mean gonado-somatic index was 9.23 ± 1.39 (1.95-21.85).
| Parameter | Mean ± SE (n = 40) | Minimum | Maximum |
|---|---|---|---|
| Length (cm) | 27.46 ± 1.47 | 12 | 38.5 |
| Weight (g) | 391.26 ± 17.92 | 144.6 | 553.4 |
| Gonad Weight (g) | 4.77 ± 0.33 | 3 | 10.5 |
| Number of eggs | 1176.32 ± 138.16 | 50 | 3000 |
| Fecundity | 14892.31 ± 2854.20 | 3257.14 | 76377.36 |
| Relative fecundity | 49.82 ± 9.87 | 6.37 | 176.86 |
| Gonadosomatic index | 9.27 ± 1.39 | 1.95 | 21.85 |
| Condition factor | 5.99 ± 1.69 | 0.71 | 30.1 |
Table 1: The mean fecundity and mean relative fecundity values of C. anguillaris.
Relationships of the characteristics of C. anguillaris at Oguta lake
The length-weight, fecundity-weight, fecundity-length, of C. anguilaris in Oguta Lake is shown in Table 2 and Figures 2-6. The linear relationship between length and weight of C. anguillaris at Oguta Lake had a coefficient of determination (R2) of 0.008, and Pearson’s correlation coefficient (r) of -0.091. The linear relationship of length and weight was not significant (p>0.05). The logarithmic linear equation relating the weight to the length was Log W=2.6772-0.0776LogL (i.e. W=475.55L-0.0776) implying a negative allometric growth pattern as the length-weight relationship coefficient of growth was -0.0776 (Figure 2).
| Relationships | Logarithmic equation (Log Y=Loga+bLogX) | A | B | Pearson’s correlation (r) | R2 | S Sig. (p-value) | Pattern |
|---|---|---|---|---|---|---|---|
| Length (L)-Weight (W) | LogW=2.6772–0.0776LogL | 475.55 | -0.0776 | -0.091 | 0.008 | 0.578ns | Negative allometric |
| Fecundity (F)-Weight(W) | LogF=5.7816–0.6889LogW | 604783.6 | -0.6889 | -0.357 | 0.127 | 0.062ns | Negative allometric |
| Fecundity(F)-Length (L) | LogF=3.0442+0.7021LogL | 12.98 | 0.7021 | 0.344 | 0.118 | 0.073ns | Negative allometric |
| Relative Fecundity(RF)-Weight(W) | LogRF=5.7769–1.6869LogW | 56.74 | -1.6869 | -0.685 | 0.47 | 0 | Negative allometric |
| Relative Fecundity (RF)-Length (L) | LogRF=0.3687+0.7920LogL | 0.1 | 0.792 | 0.301 | 0.091 | 0.119ns | Negative allometric |
Table 2: Linear relationships of the characteristics of C. anguillaris at Oguta Lake.
The fecundity-weight relationship of C. anguillaris at Oguta Lake was also not significant (p=0.062) with a Pearson’s correlation coefficient (r) of -0.357 and coefficient of determination (R2)=0.127. The fecundity-weight relationship was negative allometric with logarithmic linear equation, Log F=5.7816-0.6889LogW (Figure 3). The fecundity-length relationship was also not significant (r=0.344, R2=0.118, p>0.05) with a logarithmic equation Log F=0.0442+0.7021LogL (Figure 4).
There was a negative linear relationship between the condition factor and gonado-somatic index of C. anguillaris at Oguta Lake (Figure 7). The variations in condition factor of C. anguliaris at Oguta Lake was 19.96% (R2=0.1996) explained by the variations in gonadosomatic index.
C. anguillaris length related changes in condition factors, gonadosomatic indices and relative fecundity at Oguta Lake were shown in Figures 8-10. The mean condition factor of the fish with length between 10 and 14.5 cm was 21.67±1.20; 6.33 ± 0.81 for 15 cm-19.5 cm long fish and declined to 1.27 ± 0.12, 1.34 ± 0.18, 1.11 ± 0.07 and 0.89 ± 0.03 for length intervals 20 cm-24.5 cm, 25 cm-29.5 cm, 30 cm-34.5 cm and 35 cm-39.5 cm respectively (Figure 8).
The mean gonad-osomatic index for fish with length 10 cm-14.5 cm was (3.58 ± 0.92)%; (2.43 ± 0.48)% for 15 cm-19.5 cm long C. anguillaris and exponentially increased to (19.99 ± 0.98)% and (19.87 ± 1.08)% for length intervals of 20 cm-24.5 cm and 25 cm-29.5 cm respectively (Figure 9). For length interval of 30 cm-34.5 cm and 35 cm-39.5 cm the GSI declined to (10.45 ± 2.70) and (5.80 ± 1.05) % respectively.
The relative fecundity followed a similar trend as the gonadosomatic index of C. anguillaris at Oguta Lake (Figure 10). The relative fecundity was low at the lower length interval and peaked at between 20 cm-24.5 cm fish length.
Growth and reproductive efficiency are predictors of fish health and species fitness. Factors that interfere with reproduction and growth either directly by causing mortality or indirectly as disease agent may be deciphered by regular fish stock assessment. The length-weight relationship, fecundity and gonad-osomatic index are factors employed for assessment of health status of fish species. From the present study, the observation of negative allometric growth of the C. anguillaris in Oguta Lake is similar to observation that was made for same fish species in Ebonyi River in Nigeria [11] and Jabal Awlya Dam Lake in Sudan [12]. While Ude et al. [13] reported a negative allometric growth for C. anguillaris; however, their regression analysis for length-weight relationship showed a significant relationship between both parameters (p<0.05) unlike our observation where there was no significant association in the length-weight relationship of C. anguillaris (p>0.05). Ude et al. [13] found a significant association between the weight and length of C. anguillaris in Oguta Lake. The observation of nonsignificance in the length-weight relationship from our study may have arisen from the sample size used.
Fecundity has been reported to show association to length and weight of some fish species in some rivers [14,15]. In the present study, fecundity was affected by fish length and weight, but they were not significant. Shifa [14] reported a significant association between fish species weight and length in Dai Lake Kashmir. There was however, a significant association between relative fecundity of C. anguillaris and fish length in Oguta Lake. Hassain et al. [15] made a similar observation for Puntiusticto in Ganges River, Northwestern Bangladesh. Environmental factors such as pollution, food availability and disease affect fecundity [14]. The rate of disease is known to be dependent of age of organisms which in fish maybe estimated by fish length and weight; thus the association between length and fecundity may be linked to the environment as well. This probably accounted for the decline in C. anguillaris condition factor as the fish length increased.
The GSI of some fish species have been reported to improve as the fish mature [16]. There was an increase in length and weight which are signs of maturity and this brought about the development of the gonads, thus improving the GSI of the fish. So our observation about the association between fish length and GSI agreed with general expectations. Also, the drop in GSI after a certain length might be ascribed to a drop in egg production at old age. Thus, the negative linear relationship between GSI of C. anguillaris and condition factor is well accounted for by environmental factors and C. anguillaris assocated factors.
The mean condition factor, K value of 5.99 ± 1.69 for C. anguillaris was greater than one which suggested that the fish was in good condition. Wotton [17] reported that fish with significant K effects showed healthier wellbeing than fish with lower K values. The condition factor of fish of this study agreed with the result recorded by Idowu [18]. The high value of K recorded in this study was attributable to the feeding intensity of the fish. Oso [19] observed reduced ‘K’ effects for C. gariepinus in Ero reservoir. During the research, other fish species were observed and this suggests that there must have been competition between the spaces for food and other material needed for proper growth or for the robustness of the studied fish. Also, the high condition factor recorded in this sturdy could be attributed to increased food availability occasioned by flooding, favourable environmental condition and gonad development. Conversely, the low condition factor observed may be attributed to physiological stress due to changes in physical and chemical conditions of the habitat. Earlier researchers [20-23] made similar observation.
C. anguillaris from Oguta Lake showed a negative allometric growth pattern which implies that the fish species is increasing in length faster than in its weight which could be as a result of the polluted nature of the Lake, shortage of food supply and disease, which might have altered the pattern or process of the fish species. The fecundity of the fish as well had a negative linear relationship with length C. anguillaris in Oguta Lake. Actions should be taken to improve the health status of C. anguillaris in Oguta Lake.