Fungal Genomics & Biology
Open Access
ISSN: 2165-8056
ISSN: 2165-8056
Research Article - (2024)Volume 14, Issue 1
Objective: Our study aims to determine the epidemiological and clinical management of deep facial mycosis cases.
Methods: We have conducted a prospective descriptive study of facial sporotrichosis and chromoblastomycosis cases at the two dermatology departments of Antananarivo university hospital to document current various forms of facial infections.
Results: During a period of five years, from 2013 to 2017, 148 cases of subcutaneous implantation fungal infection were founded, including 63 cases (42.6%) of sporotrichosis and 50 cases (33.9%) of chromoblastomycosis. Nine cases (6%) on the face have been recorded, 5 of which are children aged 5 to 15. Infections are mostly located on the palpebral region, affecting 80% of the screened patients. All patients were treated orally with itraconazole.
Conclusion: Subcutaneous mycoses mainly affect children, either by self-inoculation or by co-infection of other members of the family. The lesions are serious with severe aesthetic damage. Itraconazole is an effective treatment in both adults and children.
Chromoblastomycosis; Dermo-epidermal fungal infections; Sporotrichosis; Tropical diseases
Sporotrichosis and chromoblastomycosis are implantation fungal infection; the prevalence of these diseases is higher in tropical and subtropical countries including Madagascar. It is caused by a dimorphic fungus of the genus Sporothrix [1]. Chromoblastomycosis (CBM) is a chronic fungal infection caused by dematiaceous fungal species, its causal agents are Cladophialophora carrionii, Rhinocladiella aquaspersa, Fonsecae pedrosoi, Fonsecae monophora, Fonsecae nubica [2]. Madagascar was considered to be one of the endemic country of CBM described in the world with up to 1323 confirmed cases diagnosed between 1955 and 1995. The two mains species in Madagascar are Cladophialophora carrionii and Fonsecae nubica [3]. During March 2013–June 2017, demographic and clinical chromoblastomycosis study revealed that the largeproportion of patients was farmers (62%) and lesion located principally on legs (80%) and arms (12%). Since 2013, a crosssectional study of sporotrichosis in dermatology center of Antananarivo, documented that farmers (52.4%) were principally affected and the site was predominantly on the arms (49.2%). These diseases are caused by fungi transmitted subcutaneously in humans through soil contamination or through accidental wounding by a plant material or a splinter. The majority of the patient infected lives and works in rural areas, which is permanently exposed to wood and plants traumatism. Facial mycoses are quite rare and principally affect children, often resulting in major disfigurement. Our study aims to determine the epidemiological and clinical management of deep facial mycosis cases.
We have conducted a prospective descriptive study of all cases of deep mycoses recorded at the two dermatology departments of Joseph Raseta Befelatanana university hospital. The study spanned over a period of five years from March 2013 to December 2017. Cases were referred or were automatically admitted to these two departments or they were diagnosed during field missions in endemic areas. We have included in this study all cases of confirmed facial sporotrichosis and chromoblastomycosis in both gender and all age groups. A case is confirmed when the clinical appearance is a cutaneouslymphatic or fixed cutaneous form and the mycological examination by culture and Polymerase Chain Reaction (PCR) is positive. Each clinically suspected case was subjected to skin biopsy followed by examination on Sabouraud agar with chloramphenicol at 37°C. Direct microscopic examination was performed one to two weeks later, showing yeast form with narrow base surrounded by asteroid bodies. Examination by PCR was performed using specific primers and partial sequencing to confirm diagnosis and the Matrix-Assisted Laser Desorption Ionisation-Time of Flight (MALDI-TOF) testing was used to identify the species. Pathological examination was conducted to rule out differential diagnoses, namely of tuberculosis and skin cancer. Studied parameters include age, gender, localization, clinical presentation and mycological results. We carried out clinical and biological comparison with a team of mycology experts from France on a monthly basis. Infections were treated with Itraconazole, using doses of 100 mg twice per day, for at least six months.
Out of the 148 mixed cases of deep mycoses (sporotrichosis, chromoblastomycosis, mycetoma), we have identified 63 (42.5%) cases of sporotrichosis caused by agent Sporothrix schenckii, including 3 cases of facial infections (Table 1) and 50 (33.9%) cases of chromoblastomycosis with 2 cases of facial infections (Table 1). Three cases were found in children aged respectively 4, 5 and 10. On average, lesions start to develop a month and a half before consultation. Only one case presented a trauma prior to the appearance of lesions. Infections are mostly located on the palpebral region, affecting 80% of the screened patients (Figure 1). One case had both ulcerous, scabby lesions on the face and cutaneous-lymphatic lesions from the left hand to the arm. A case of infections among family members was observed. A child had lesions on the face and the mother had cutaneouslymphatic lesions on the arm (Figure 2). Only three of those patients have been treated. Two others were abandoned treatment. Facial sporotrichosis was very sensitive to Itraconazole dose of 200 mg administered per day during an average period of 3 to 6 months.
| Sl. No | Gender | Age | Location and clinical description | Results |
|---|---|---|---|---|
| PCR/MALDI-TOF | ||||
| 1 | Male | 6 | Fluctuating nodular lesion, along a lymphatic path of the middle of the face | Sporothrix schenkii |
| 2 | Female | 10 | Ulcerous lesion on the root of the nose | Sporothrix schenkii |
| 3 | Male | 5 | Nodular linear lesion on left cheek and cutaneous lymphatic lesion of the arm. | Sporothrix schenkii |
| 4 | Male | 46 | Erythemato-squamous atrophic scarring plaque on the lower eyelid and right cheek. | Cladophialophora carrionii |
| 5 | Male | 60 | Retractile erythematous plaque on the all right hemiface | Cladophialophora carrionii |
Note: PCR/MALDI-TOF: Polymerase Chain Reaction/Matrix Assisted Laser Desorption Ionization-Time of Flight.
Table-1: Epidemio-clinical aspects of mycoses of the face.
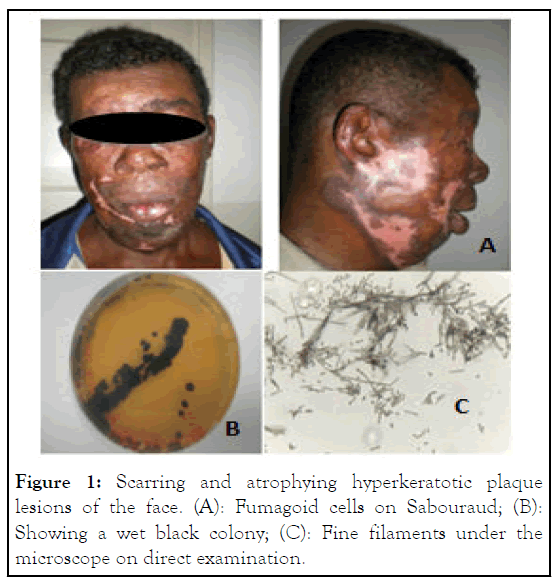
Figure 1: Scarring and atrophying hyperkeratotic plaque lesions of the face. (A): Fumagoid cells on Sabouraud; (B): Showing a wet black colony; (C): Fine filaments under the microscope on direct examination.
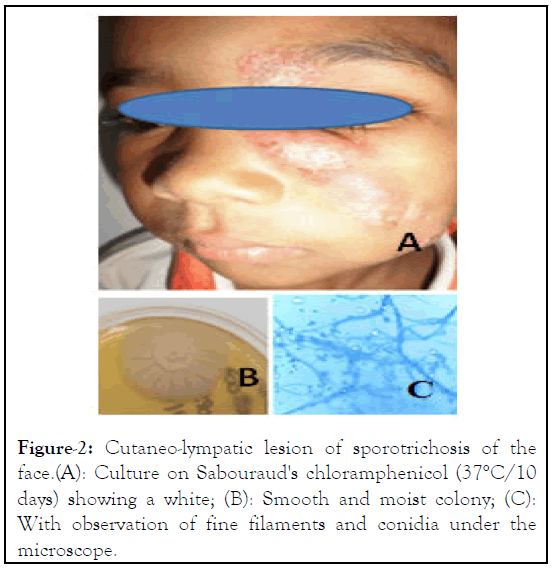
Figure 2: Cutaneo-lympatic lesion of sporotrichosis of the face.(A): Culture on Sabouraud's chloramphenicol (37°C/10 days) showing a white; (B): Smooth and moist colony; (C): With observation of fine filaments and conidia under the microscope.
Sporotrichosis and chromoblastomycosis are dermoepidermal fungal infections. They prevail in most tropical countries with a particularly higher level of endemicity in Japan, Brazil, Peru, Mexico, India, Uruguay and Madagascar [4].
Sporotrichosis is an infection found mainly in the highlands of Madagascar (Figure 2). Therefore, the lesions principally develop on the lower limbs of infected patients, mostly people walking bare feet on a permanent basis such as farmers or woodcutters. Cases of deep facial mycoses have not fully been reported in the literature, its occurrence is not totally rare. In countries with high endemicity like Brazil, facial lesions represented 7.9% of sporotrichosis infections in 2004 [5]. In Peru, studies conducted during a period of 28 years, showed that the face was the most commonly affected region (647 cases) mainly children aged ≤ 14 years [5]. In India in 2005, facial sporotrichosis constituted 26% of all cases of sporotrichosis [6].
In Peru, sixty percent of patients with deep facial mycosis represented children aged less than 14 years old. Actually, children under the age of 15 are risk cases of deep facial mycoses (p<0,001) (study performed in Brazil in 2004) [7]. School-aged children between are the most infected because they play in playgrounds, gardens, open grounds and get hurt quite easily. Our study showed similar results with an infection rate of 83.3% among pediatric patients. The use of firewood occur the risk of children infection, mainly in rural communities of Madagascar. Children regularly perform the chore of collecting firewood, during which they may easily get splinters in the skin. Their height may also explain facial infections because plants responsible for transferring fungi stand at the same height as their faces. Contamination is caused by wears short sleeves, without protective gloves or boots, for people working in rural areas in which spiked plants are common. Auto-inoculation is also a risk factor of fungi transmission on the face. In our study, we identified one boy with infections on both the forearm and the face. Inoculation through physical contact between relatives is another risk of contamination of the face. We observed a case of a little boy infected on his face and his mother, who presented a cutaneous-lymphatic sporotrichosis in the arm [8].
Nineteen percent of cases noted trauma prior to the development of lesions. The trauma experience was sometimes overlooked or unknown, yet it significantly informs diagnosis. In our study, only one patient admitted having experienced traumatism. Clinically, sporotrichosis appeared in cutaneouslymphatic form in 55% of the cases while the fixed cutaneous form was found in 27% of the cases. Sporotrichosis lesions reinfect quite easily, subsequently becoming more inflammatory, purulent and serous. Lesions produce serpiginous swelling on the face, potentially leading to disfigurement and reducing the quality of life of patients. Sometimes, they trigger the dysfunction of organs and limbs, resulting in atrophy and mutilation [9]. Otherwise, the infection can occur after local or hematogenous dissemination, local spread can occur when overlying skin lesions progress resulting in underlying erosive bone disease [10]. Face location is also dangerous and the diagnosis must be done quickly for avoiding complications.
According to the literature, facial chromoblastomycosis concerns principally adults. The age more than 33 years was 5 times higher risk for having chromoblastomycosis (Odds Ratio (OR) 5.44, 95% CI: 2.04-17.10; p=0.0001). The main location is legs, it was demonstrated that, a diagnosis of chromoblastomycosis was 3 times more likely than any other diagnoses for leg lesions (OR: 3.36, 95% CI: 1.45-8.4; p=0.003). Nevertheless, face, head and neck locations were described in 158 cases about 7.740 world chromoblastomycoses cases (2%) [11]. It appeared as nodular, warty hyperkeratosic plaques or scabby plaques. Other clinicals presentation forms of implantation fungal infection are characterized by atrophic lesions evolving to mutilation in facial orifice lesion, showed by our patient. By differentiate of sporotrichosis, chromoblastomycosis lesions are more serious with various cases of mutilation in advanced stage. Facial implantation mycosis often leads to misdiagnosis, responsible for eyelid retraction, several grades of ectropion, xerophthalmia and keratitis. Brain abscesses cases are reported caused by F. monophora and F. pugnacious. During therapy, chromoblastomycosis lesions may present an intense fibrotic reaction resulting in scarring. Pathological examination should help avoid differential diagnoses. Differential diagnoses mainly include skin tuberculosis, lupus vulgaris, epithelial carcinoma and mycetoma. However, it is advisable to presume a case of deep mycosis at the sight of chronic ulcerous lesions.
The effectiveness of Itraconazole was described for deep facial mycosis in our study, for a dose of 200 mg per day during a period of 3 to 6 months, for adults. Itraconazole showed very positive treatment responses with good therapeutic tolerance on both adults and children. On pediatric patients, based on a Chinese meta-analysis in 2018, itraconazole is a drug of choice to treat sporotrichosis with a dose of 3-5 mg/kg/day during a period of 3 to 6 months [12]. Terbinafine is another effective drug for the treatment of chromoblastomycosis. Unfortunately, the cost of these drugs is quite high for the general public in a developing country like Madagascar.
Facial sporotrichosis and chromoblastomycosis are rare cases of mycosis that are difficult to diagnose. These two mycoses are endemic in Madagascar, especially in hot and humid zones, but they are still neglected and the access of treatment is limited. It is then advisable not to rule them out when encountering chronic and inflammatory, ulcerous lesions, particularly on children. Being children, auto-inoculation and infection of other family member are risks factors for facial contamination. Early treatment could inhibit the expansion of the pathology. Treatment with itraconazole produces satisfactory of the lesion and improve the appearance results and is well tolerated, subsequently requiring reconstructive procedure if needed.
Thanks to Charles Mérieux Foundation and the Infectious diseases Center Charles Mérieux, Antananarivo, Madagascar.
MA: Research design, raw data collection, investigation, visualization, writing original draft and editing. MFR: Statistical analysis, review and editing FA: Research design, raw data collection, and review. LSR: Conceptualization, review and editing. TMR: Research design, review and editing. IMR and FRR: Research design, project administration, supervision, validation, writing-review and editing. All authors read and approved the final manuscript.
This study was open access funded by the Charles Mérieux Foundation.
The data sets used and analysed during the current study are available from the corresponding author on reasonable request.
Approval and consent to participate the study was approved by ethics committee of the ministry of public health of Madagascar.
All patients were consenting and signed a consent letter prior to the study.
Authors declare no competing interests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Andrianarison M, Rakotosaona MF, Rabenja FR, Sendrasoa FA, Rasamoelina TM, Sata M, et al. (2024) Facial Sporotrichosis and Chromoblastomycosis in Madagascar: Serial Cases. Fungal Genom Biol. 14:242
Received: 06-Mar-2024, Manuscript No. FGB-24-30007; Editor assigned: 08-Mar-2024, Pre QC No. FGB-24-30007(PQ); Reviewed: 22-Mar-2024, QC No. FGB-24-30007; Revised: 29-Mar-2024, Manuscript No. FGB-24-30007(R); Published: 05-Apr-2024 , DOI: 10.35248/2165-8056.24.14.242
Copyright: © 2024 Andrianarison M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This study was open access funded by the Charles M�©rieux Foundation.