Angiology: Open Access
Open Access
ISSN: 2329-9495
ISSN: 2329-9495
Case Report - (2022)Volume 10, Issue 3
Background: Intravascular Ultrasound (IVUS) demonstrates the positions of intervention devices and vascular structures on a cross-sectional short axis to support interventional procedures, especially for complex morphological lesions. Extravascular Ultrasound (EVUS) visualizes vessel structures and devices on the long and short axes. EVUS guided wiring reduces radiation exposure by avoiding fluoroscopy use. EVUS handling, which guides the direction in which the guidewire should go on both the long and short axes, is more complicated, stressful, and timeconsuming than IVUS. To solve this issue, we propose a new guidewire crossing method; extra and intravascular ultrasound (E&IVUS) guided wiring, which uses both simultaneously.
Materials and methods: This is the first report on E&IVUS guided wiring for femoropopliteal arterial occlusions. EVUS guided wiring requires alternating long and short axis images to locate the devices. However, when EVUS is handled to change from a short axis to a long axis image, the long axis image may differ from the original long axis image. E&IVUS dedicate EVUS and IVUS to acquiring the long axis and the short axis view, respectively. As a result, E&IVUS reduce the stress due to the manipulation of the echo probe to switch from the long to short axis image and vice versa and may shorten the guidewire crossing time. Furthermore, intimal wire tracking can be performed according to the EVUS long axis and IVUS short axis images. The case involved a 76-year-old female with right superficial femoral artery occlusion. A hard guidewire supported with an over-the-wire type of IVUS was successfully passed into the plaque of the occluded lesion while confirming their positions with E&IVUS. An appropriately sized drug-coated balloon dilated the lesions according to IVUS measurements. Finally, adequate blood flow was obtained without complications.
Conclusion: E&IVUS guided wiring is a more rational lesion-crossing strategy than EVUS or IVUS alone. The clinical outcomes with this technique need to be evaluated.
Extravascular ultrasound; Intravascular ultrasound; Chronic total occlusion; Femoropopliteal lesion; Peripheral intervention
Intravascular Ultrasound (IVUS) demonstrates the positions of intervention devices and vascular structures on a cross-sectional short axis to support the interventional procedures, especially for complex morphological lesions. Extravascular Ultrasound (EVUS) is a useful diagnostic device and a new therapeutic assistive device for guiding and monitoring the progress of peripheral interventions [1]. EVUS visualizes the devices and vessel structures in the long and short axes and reduces radiation exposure by avoiding fluoroscopy during guidewire manipulation.
The efficacy of IVUS and EVUS guided intervention for Chronic Total Occlusion (CTO) has been reported [2-4]. Both methods facilitate intraplaque tracking to yield better clinical outcomes [5-7]. However, there is a learning curve involved in viewing and understanding images of structures taken using both imaging modalities. Operating EVUS, which presents appropriate images for guidewire manipulation in both the long and short axes, is more complex and time-consuming than IVUS, which only uses the short axis image. EVUS is stressful for both the operator and sonographer. Therefore, although EVUS is a useful device, it is not yet widely used. To solve this issue, we propose a new guidewire crossing method, Extra and Intravascular Ultrasound (E&IVUS) guided wiring, which uses both simultaneously.
This is the first report on E&IVUS guided wiring for femoropopliteal arterial occlusions. EVUS guided wiring requires alternating long and short axis images to locate the devices during CTO wiring. EVUS guided wiring requires the wire to be positioned and advanced into the CTO while manipulating the echo probe from the long axis to the short axis and from the short axis to the long axis. When EVUS is handled to change from a short axis to a long axis image, the long axis image may differ from the original long axis image. E&IVUS, which combines EVUS with IVUS, dedicate EVUS and IVUS to acquiring the long axis and the short axis view, respectively. As a result, E&IVUS reduce the stress due to the manipulation of the echo probe to switch from the long to short axis image and vice versa and may shorten the guidewire crossing time. Furthermore, intimal wire tracking can be performed according to the EVUS long axis and IVUS short axis images.
The technical flow of E&IVUS guided wiring is as follows: A 6 Fr guiding sheath is inserted ipsilaterally or contralaterally. Unfractionated heparin (5000 IU) is injected intra-arterially through the sheath. An Over-The-Wire (OTW) type of IVUS (Visions PV.018 OTW, Philips) is used to visualize and analyze the lesions in the short axis and to further support the guidewire force. A hard 0.018-inch guidewire (Astato, Asahi Intec., Japan) is passed through the IVUS OTW lumen and reaches the orifice of the occlusion. A C-arm is not used and is moved away from the operative field during E&IVUS guided wiring. A sonographer stands on the opposite side of the operator to handle an echo probe. EPIQ Elite (Philips, Netherlands) with 22 MHz linear probes is used to identify the long axis image during guidewire manipulation. The probe must be fixed to show the center of the vessel, and the operator should gently control and move the guidewire while maintaining clear delineation of the guidewire in the EVUS long axis image (Figure 1).
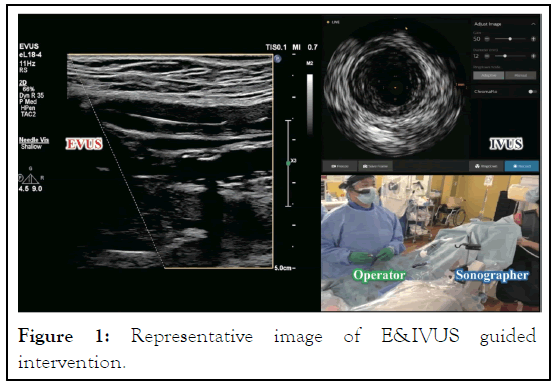
Figure 1: Representative image of E&IVUS guided intervention.
We use the needle visualization mode of EPIQ Elite. In this mode, when the guidewire tip and the echo beam overlap in the long axis direction, echo reflection occurs, and the tip of the guidewire glows (Figure 2a). By advancing the wire without deviating from the long axis of the EVUS while reflecting the tip of the guidewire, the wire can enter the plaque in the proper direction. Therefore, there is no need to check the tip of the guidewire in the EVUS short axis. For mutual understanding between the operator and the sonographer, the EVUS long axis image is divided into seven sections and given each name (Figure 2b).
Figure 2: Representative image of EVUS. (a) A guidewire tip is reflected by an EVUS beam, especially with the needle visualization mode of EPIQ Elite (Philips, Netherlands), when both directions are overlapped (yellow arrow). The guidewire can be advanced into the relative center of the lesions if the wire is advanced while maintaining this reflex of the tip of the guidewire. (b) The EVUS image on the long axis is divided into seven sections to create a mutual understanding between an operator and a sonographer.
After the guidewire is advanced from the P1 to D3 segment, IVUS follows the guidewire and confirms the guidewire position, plaque quality, and vessel size. This process is repeated until the wire crosses the occlusion. If IVUS does not follow the guidewire due to hard plaque, we should switch to EVUS guided wiring to confirm the guidewire position from the EVUS short axis image instead of the IVUS short axis image. After guidewire crossing, IVUS is subsequently passed through the lesion, and the hard guidewire is replaced with a soft guidewire through the IVUS OTW lumen. The lesion is then evaluated with IVUS. Finally, the lesion is revascularized with appropriately sized balloons and scaffolds from the IVUS analysis.
We present a representative case of treatment with E&IVUS guided wiring. A 76-year-old female with severe claudication had a long right Superficial Femoral Artery (SFA) (Figure 3a). Astato supported with Visions PV.018 OTW was advanced to the CTO orifice. EVUS confirmed their positions in the long axis after the C-arm was moved away. We gently manipulated the guidewire to enter the CTO under EVUS guidance. The guidewire reached the D3 segment in the EVUS long axis, and then IVUS followed the guidewire to ensure that the guidewire route was in the intima and to assess the plaque quality and vessel size. We repeatedly performed this process, and all intimal tracking through the occlusion succeeded (Figure 3b). Drugcoated balloon appropriately sized by IVUS measurement dilated the lesions. Finally, sufficient lumen area and blood flows were achieved without complications (Figures 3c and 3d).
Figure 3: Representative case of E&IVUS guided intervention. (a) Control angiography of right superficial femoral arterial occlusion. (b) IVUS images of all intimal tracking with the E&IVUS procedure. (c) IVUS images of a sufficient minimal lumen area after drug-coated balloon dilatation. (d) Final angiography after E&IVUS guided intervention for superficial femoral arterial occlusion. Periprocedural complications, restenosis, reinterventions, and limb loss were observed one year after the procedure.
In terms of limitations of E&IVUS are here. First, IVUS resonates with the frequency of EVUS, which causes noise in the IVUS image. To solve this noise, adjusting the depth of the IVUS image eliminates the effects caused by the noise. Second, the parallel wire technique not using a microcatheter can be performed, but this wire technique using a microcatheter is impossible with a 6 Fr sheath due to Visions PV.018 OTW of 5 Fr. If this technique is conducted, a 7 Fr sheath or another thinner type of IVUS should be used. Third, calcified vessels and lesions are poorly visualized with EVUS due to acoustic shadow caused by the inability of ultrasound to penetrate behind the calcification boundary. Fourth, the popliteal artery, which runs dorsal to the knee, is difficult to delineate by EVUS in the normal supine position. Therefore, the leg position needs to be changed to the frog position. Finally, it is difficult to use in obese patients where the depth from the surface of the foot to the blood vessels is so deep that echo ultrasound cannot reach them. We should recognize the limitations of EVUS and change the treatment strategy.
E&IVUS guided wiring is a more rational lesion-crossing strategy than EVUS or IVUS alone. Both devices can more accurately visualize what is happening in the blood vessels during wiring, and their use during procedures has been reported to be associated with improved clinical outcomes. As peripheral vascular intervention becomes more complex, the accuracy of the guidance instruments used during the intervention is related to clinical and technical success. This technique is expected to reduce the amount of contrast media used and radiation exposure because the C-arm is not used during CTO wiring under E&IVUS guidance. Furthermore, this technique can perform intraplaque wiring and may contribute to the safety of the procedure, lower periprocedural adverse events, and better clinical outcomes. Therefore, the clinical effects of this technique need to be evaluated.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Haraguchi T, Sawada N, Tsujimoto M, Kashima Y, Sato K, Fujita T (2022) Extra and Intravascular Ultrasound (E&IVUS) Guided Wiring for Femoropopliteal Arterial Occlusive Lesions. Angiol Open Access. 10:287.
Received: 20-Sep-2022, Manuscript No. AOA-22-19036; Editor assigned: 23-Sep-2022, Pre QC No. AOA-22-19036 (PQ); Reviewed: 07-Oct-2022, QC No. AOA-22-19036; Revised: 14-Oct-2022, Manuscript No. AOA-22-19036 (R); Published: 21-Oct-2022 , DOI: 10.35248/2329-9495.22.10.287
Copyright: © 2022 Haraguchi T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.