International Journal of Physical Medicine & Rehabilitation
Open Access
ISSN: 2329-9096
ISSN: 2329-9096
Research Article - (2023)Volume 11, Issue 4
Background: Peripheral neuropathy is a common neurological disease with numerous causes. Diabetes remains one of the most common causes with 50% of diabetic patients affected at some stage of disease progression. To identify and screen potential small molecule therapeutics effectively, sophisticated pre-clinical models are needed. In-vitro models provide a useful way to explore the fundamental effects of potential therapies and aid the understanding of their mechanistic properties.
Methods: Within this study we have developed an in-vitro model of neurodegeneration, which was then used to screen the effect of the B vitamins (B1, B6 and B12) on neurite regeneration.
Findings: Our results indicate vitamins B1, B6 and B12 in combination or individually improve neurite regeneration in NG108-15 neural cells after they have been insulted with hydrogen peroxide to induce degeneration. This beneficial effect on neurite extension was seen with both pre and post-insult treatment. Each B vitamin showed an individual beneficial effect on neurite regeneration but to varying degrees.
Conclusion: We have established a novel in-vitro model of neurodegeneration which can be used as a tool to screen potential therapeutic compounds and using this model, we provide evidence of the neurite regenerative capacity of vitamins B1, B6 and B12.
Peripheral neuropathy; Neurodegeneration; Vitamins B1, B6 and B12; Neural cells; 3D Culture
Peripheral neurodegeneration, the functional deterioration of the nerves outside the brain and spinal cord, is a consequence of various causes, contributing to poor patient quality of life [1]. Peripheral neuropathies are among the most common neurological diseases resulting from a wide spectrum of clinical syndromes including immune-mediated conditions, toxic substances such as alcohol and chemotherapy, vitamin B deficiencies, viral infections, inflammatory disorders, and metabolic disorders such as diabetes [2-4]. Diabetes is the most common cause of peripheral neuropathy, with 50% of diabetic adults affected during their lifetime [5]. Diabetic peripheral neuropathy can result in pain, paraesthesia, foot ulcers and, in severe cases, lower limb amputation [6]. Alongside aggressive screening and lifestyle alterations, the development of preventative treatments is crucial for reducing the severity and prevalence of peripheral neuropathy [7-11].
Modelling neurodegeneration and subsequent regeneration allows potential regenerative and neuroprotective compounds to be screened, which could be applied to the treatment of diabetic peripheral neuropathy [12-14]. In-vitro models play a crucial role in this because they can be specifically designed to recreate the phenotype of interest in an uncomplicated system where cell behavior can be easily observed [1,12]. One in-vitro approach to modelling neurodegeneration is monolayer cell culture. Monolayer systems have distinct advantages which facilitate their ability to give essential insights into the therapeutic efficacy of compounds. However, due to culture upon flat stiff plastic, monolayer cells are forced to adhere in an unnatural environment. This limits their exposure to the spatial and mechanical cues present within their natural environment thereby affecting growth, function, and morphology [15]. This limitation can be somewhat mitigated by advancing to 3D coculture models, where cells are cultured on or within materials similar to those found in-vivo, alongside other cell types which are known to influence behavior, enabling the exploration of cell-tocell interactions [16, 17]. Such 3D cell co-culture models can be advanced further still by perturbing the system to study cell damage, and the subsequent recovery, in the presence and absence of therapeutics [18]. Some examples of recent 3D culture models for peripheral nerve cell systems can be found in the literature [19-24]. Here, our previous model designed for the screening of drugs to improve regeneration after nerve injury [25] was adapted to establish a novel neuropathy model with applications in neurodegenerative and neuroprotective compound screening. The induction of neurodegeneration can be achieved through multiple different methods, including the use of hypoxia, or chemical agents such as paclitaxel, suramin and hydrogen peroxide [26-35]. Following this neurodegenerating insult, outcome measures such as neurite extension indicate the impact of the insult and any beneficial effect of a compound administered before or after the insult. Our model comprises of an anisotropic cellular hydrogel containing highly aligned Schwann cells co-cultured with a neural cell line [36]. The model enables the effects of chemical insult on neurites to be studied in the presence of aligned extracellular architecture. Upon model optimization, the recovery of the neural cells from the neurodegenerative insult was used for the investigation of a potential neuropathy therapy, a combination of B vitamins [37-40]. All B vitamins contribute to physiological functions, but some are known to be neurospecific, playing important roles in the health of the nervous system, particularly vitamins B1, B6 and B12 [41,42]. Studies have demonstrated the benefit of vitamin B treatment in several neurological diseases such as Wernicke's encephalopathy, depression, beriberi, seizures, and peripheral neuropathy [43-50]. Moreover, studies have identified synergistic properties of vitamins B1, B6 and B12 to improve peripheral neuropathy, motor function, and pain [42,43,51-53]. Therefore, the aim of our study was to develop a novel in-vitro model of neurodegeneration and utilize it to explore the neurite regenerative and neuroprotective capacity of vitamins B1, B6, and B12.
All materials were obtained from Sigma-Aldrich, UK unless otherwise stated.
Cell Cultures
SCL4.1/F7 cells (immortalized rat Schwann cell line (Health protection agency, UK)) were grown in culture medium (high glucose Dulbecco’s Modified Eagle Medium (DMEM)) supplemented with 10% v/v Foetal Bovine Serum (FBS) and 100 U/mL of Penicillin, 100 µg/mL of Streptomycin, in standard cell culture flasks. The cultures were maintained at subconfluency at 37°C with 5% CO2 and passaged when required. NG108-15 cells (mouse neuroblastoma × rat glioma hybrid neural cell line, (Merck)) were expanded in culture medium (DMEM: F12 (Thermo Scientific)) supplemented with 100 U/ mL of Penicillin, 100 µg/mL of Streptomycin and with 10% v/v FBS in standard cell culture flasks. The cultures were maintained at sub-confluency at 37°C with 5% CO2 and passaged when required. When used in assays to determine neurite growth, FBS was removed.
Preparation of medium with and without B vitamins
DMEM: F12 medium lacking vitamin B1, vitamin B6, and vitamin B12 was prepared (all other vitamins remained); for 500 mL of medium 2250 mg D-glucose, 52 mg L-tyrosine disodium salt dehydrate, 21 mg L-serine, 15 mg glycine, 3.6 mg myoinositol, 2 mg calcium-d-pantothenate, 2 mg choline chloride, 2 mg folic acid and 0.05 mg iron (III) nitrate nonahydrate were added to 465 mL Earle’s Balanced Salt Solution (EBSS) (Merck) containing 20 mL Minimum Essential Medium Eagle (MEM) (Merck) (10X), 10 mL L-glutamine (200 mM) (Thermo Scientific), 100 U/mL penicillin and 100 µg/mL streptomycin. This medium is referred to as ‘vitamin B free’ throughout the manuscript. B1, B6 and B12 vitamins were added individually or in combination as indicated. Vitamin B combination refers to a dose of 40 µM vitamin B1, 20 µM vitamin B6, and 0.4 µM vitamin B12 as used previously in-vitro on healthy mouse dorsal root ganglion (DRG) neurons [54].
Seeding for monolayer cultures for Incucyte analysis
NG108-15 cells were seeded at a density of 5,000 cells/well onto a 96-well plate in vitamin B free and FBS free medium. The plate was loaded into an Incucyte S3 Live-Cell Analysis system (Sartorius) and analyzed every 1-4 hours to monitor neurite growth using phase-contrast imaging. Automated neurite length analysis was conducted using the Incucyte Neuro Track analysis module.
Fabrication of 3D EngNT co-cultures
Anisotropic cellular gels were prepared using 1 mL of solution containing; 80% v/v Type I rat tail collagen (2 mg/mL in 0.6% acetic acid) (First link UK Ltd), 10% v/v minimum essential medium (MEM), 5.8% v/v sodium hydroxide and 4.2% Schwann cell suspension (2-4×106 SCL4.1/F7 cells per 1 mL gel) and was integrated with tethering mesh at opposite ends of a rectangular mould [55]. Cellular gels were immersed in 10 mL medium and incubated at 37°C with 5% CO2 overnight (minimum 16 h) to enable cellular self-alignment. These gels were then stabilized using RAFT™ absorbers (Lonza) which were placed directly on top of the gel for 30 seconds. Each stabilized aligned cellular gel was cut into 4 equal segments to allow higher throughput of treatment screening. A small notch was made in the side of each gel to distinguish the orientation for future analysis. Each gel segment was transferred to a separate well in a 24-well plate, then 100,000 NG108-15 cells, in vitamin B free and FBS free medium, were seeded on top of each segment for co-cultures.
Insults
For model development, monolayer cell cultures were subjected to four separate insults for 24 hours: hydrogen peroxide (1-400 µM); suramin (20-400 µM) (Merck); paclitaxel (30-1000 nM) (Merck); or hypoxia (1% oxygen maintained using a hypoxia incubator (HypoxyLab, Oxford Optronix)). When screening the effects of vitamin B1, B6 and B12 in monolayer or 3D cocultures, they were subjected to 5-10 µM hydrogen peroxide for 2-4 hrs. Following the insult, the medium was removed and replaced with either vitamin B free medium or medium supplemented with vitamin B1, B6 and/or B12.
Immunocytochemistry
Following fixation overnight of the co-cultures with 4% (w/v) paraformaldehyde (PFA), gels were washed 3 times with phosphate buffered saline (PBS). Gels were then simultaneously permeabilized and blocked with 5% blocking serum in a solution of 0.5% Triton-X-100 in PBS. Gels were washed before the addition of primary antibody (anti-βIII-Tubulin, Invitrogen UK) diluted (1:400) in PBS and incubated overnight (minimum 16 h) at 4°C. Washes were repeated before adding the corresponding secondary antibody (Dylight anti-mouse IgG 488 or 549) diluted (1:200) in PBS for 90 minutes at room temperature. The secondary antibody was washed off and the samples were stored at 4°C in PBS before viewing.
Image analysis and quantification
Phase contrast images were captured using the Incucyte S3 at 20x magnification and fluorescence microscopy (Zeiss Axiolab A1, Axiocam Cm1) was used to capture images of neurites from predetermined fields of each gel using a 20x lens. All neurites in the determined fields were manually measured using Image J [56].
Statistical analysis
Normality tests were conducted on all data to determine appropriate statistical tests, and an unpaired two-tailed T-Test, mixed effects analysis, one-way Analysis of Variance (ANOVA) or two-way ANOVA was performed, as data followed a normal distribution. One-way ANOVA was followed by a Dunnett’s post hoc test, Two-way ANOVA with Bonferroni. For all tests, *p<0.05, **p<0.01, ***p<0.001 and ****p<0.0001 were considered to be significant.
Degeneration model development
To advance our previously developed 3D-engineered co-culture nerve regeneration model to a degeneration model, a method to induce neurite degeneration had to be established. To achieve this, multiple insults were tested on our chosen cell line, NG108-15, and the cells were monitored over time for neurite degeneration. However, to quantify neurite degeneration we first needed to establish baseline growth of neurites, which can be initiated by withdrawal of FBS from the media [57]. Further to this, as this model’s intended use was to investigate the effect of B vitamins, there was the additional challenge of establishing the baseline level of neurite growth in the absence of vitamins B1, B6 and B12. As standard commercially available cell culture medium contains basal levels of B vitamins, we manufactured our own medium that contained no vitamin B1 (thiamine hydrochloride), B6 (pyridoxal hydrochloride) and B12 (cyanocobalamin). NG108-15 cells were able to extend neurites in this vitamin B1, B6 and B12 free medium for up to 4 days in the monolayer environment. After this period, the viability of the cells appeared to decrease significantly (Figures 1A-1D). The reduced viability of the cells was only observed in the absence of FBS. However, prior to this, neurite extension was greater in the absence of FBS (Figures 1B-1E). Neurite growth was also seen in the 3D co-culture system in the absence of vitamin B1, B6 and B12 and FBS (Figure 1I-1J). Once neurite growth was established in vitamin B1, B6 and B12 free culture medium, the timeframe in which to monitor NG108-15 neurite extension was investigated by assessing neurite growth every 4 hours in monolayer culture. Neurite growth did not change significantly between 24 to 48 hours (Figures 1F-1H), therefore, to progress the model towards higher throughput, a 24-hour time point was chosen. Different seeding cell densities were also tested to ensure optimal cell seeding for growth and neurite extension; a cell density of 5,000 cells per well in a 96-well plate format was identified as the optimal.
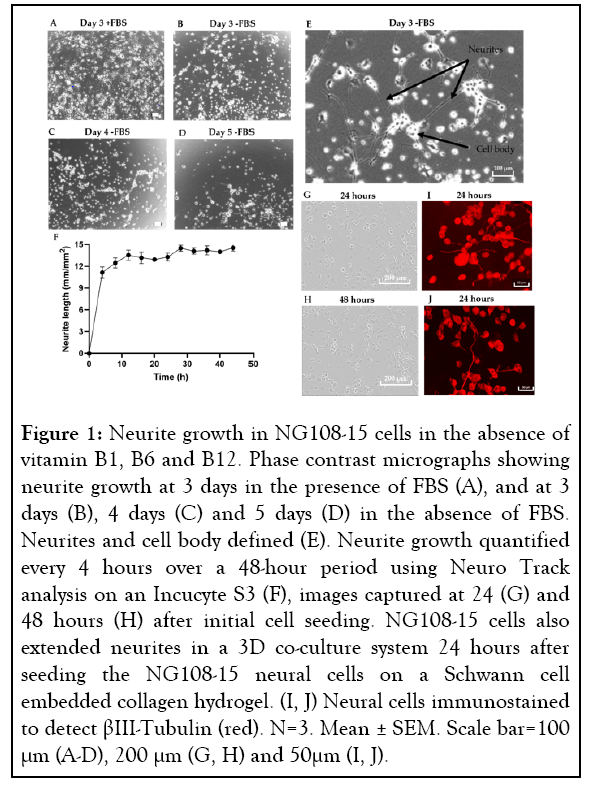
Figure 1: Neurite growth in NG108-15 cells in the absence of vitamin B1, B6 and B12. Phase contrast micrographs showing neurite growth at 3 days in the presence of FBS (A), and at 3 days (B), 4 days (C) and 5 days (D) in the absence of FBS. Neurites and cell body defined (E). Neurite growth quantified every 4 hours over a 48-hour period using Neuro Track analysis on an Incucyte S3 (F), images captured at 24 (G) and 48 hours (H) after initial cell seeding. NG108-15 cells also extended neurites in a 3D co-culture system 24 hours after seeding the NG108-15 neural cells on a Schwann cell embedded collagen hydrogel. (I, J) Neural cells immunostained to detect βIII-Tubulin (red). N=3. Mean ± SEM. Scale bar=100 μm (A-D), 200 μm (G, H) and 50μm (I, J).
Neurotropic effect of the combination of vitamins B1, B6 and B12 on healthy NG108-15 neural cells
This model was then used to analyze neurite growth in the presence and absence of a combination of vitamins B1, B6 and B12. The concentrations of the B vitamins used were based upon a previous in-vitro study investigating the impact of vitamins B1, B6 and B12 on neurite growth and nerve cell network of healthy mouse DRG neurons [54]. Over a 72-hour period, longer neurite length was recorded (p=0.0013) when the cells were grown in the medium supplemented with vitamins B1, B6 and B12 in comparison to the vitamin B free medium (Figure 2).
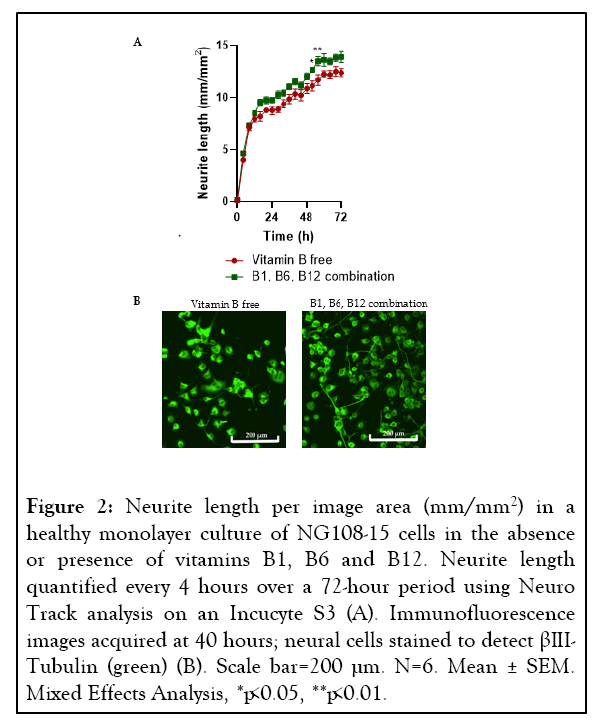
Figure 2: Neurite length per image area (mm/mm2) in a healthy monolayer culture of NG108-15 cells in the absence or presence of vitamins B1, B6 and B12. Neurite length quantified every 4 hours over a 72-hour period using Neuro Track analysis on an Incucyte S3 (A). Immunofluorescence images acquired at 40 hours; neural cells stained to detect βIIITubulin (green) (B). Scale bar=200 µm. N=6. Mean ± SEM. Mixed Effects Analysis, *p<0.05, **p<0.01.
Insults to induce neurite degeneration in NG108-15 cells
Four approaches were used to induce neurite degeneration: exposure to hypoxia, treatment with suramin, the chemotherapeutic agent paclitaxel and hydrogen peroxide (H2O2). Our study found that exposing the NG108-15 cells to hypoxic conditions had no effect, therefore this insult was disregarded for further use in the study. The remaining three approaches caused degeneration of the neurites without causing cell death. A dose response to determine the neurite degenerative effect of suramin produced conflicting results. Higher doses of suramin appeared neurotoxic and caused substantial degeneration of the neurites, whereas at lower doses (20 µM) the neurite growth was higher than that seen in the control group, which received no insult (Figure 3A). For this reason, it was decided not to use suramin for further studies. Paclitaxel caused neurite degeneration which occurred rapidly and continued to decline over 72 hours at any dose the cells were exposed to, therefore, this approach was also discarded for the model (Figure 3B). The final approach to induce degeneration was treatment with H2O2. Previous literature had shown treatment with 400 µM H2O2 for 24 hours induced degeneration in neuronal cells [35]. When we tested this dose, we found it was neurotoxic as it not only degenerated the neurites but also reduced cell viability. The same was seen with a 10-fold lower dose of 40 µM H2O2. Another study in the literature indicated that much lower doses of H2O2 were needed to degenerate the neurites without reducing the viability of the cells [58]. We tested further doses of H2O2 (1-10 µM), and degeneration was only observed in concentrations 5-10 µM (Figure 3C). A 1 µM dose exhibited no degeneration and so it was decided 5-10 µM H2O2 was optimal to induce degeneration in these cells (Figure 3C).
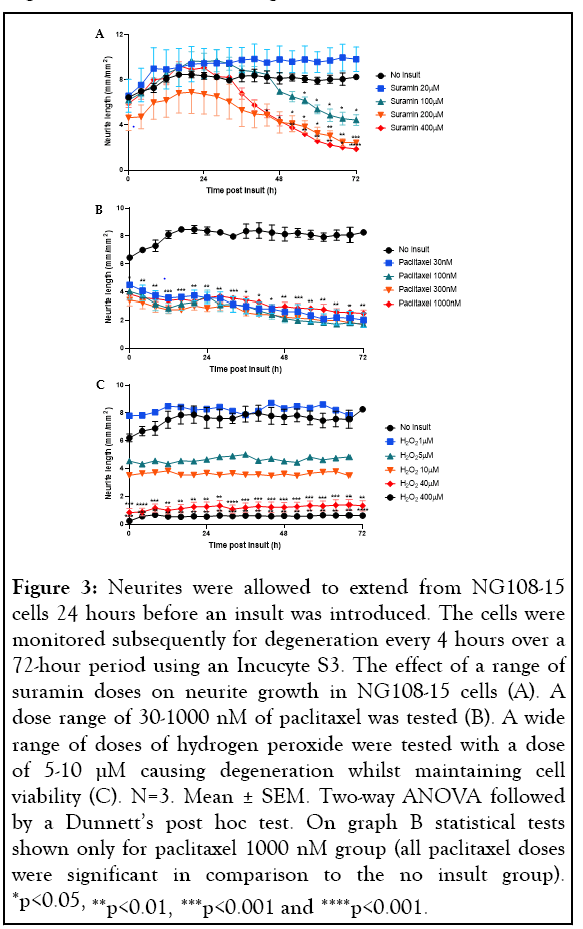
Figure 3: Neurites were allowed to extend from NG108-15 cells 24 hours before an insult was introduced. The cells were monitored subsequently for degeneration every 4 hours over a 72-hour period using an Incucyte S3. The effect of a range of suramin doses on neurite growth in NG108-15 cells (A). A dose range of 30-1000 nM of paclitaxel was tested (B). A wide range of doses of hydrogen peroxide were tested with a dose of 5-10 µM causing degeneration whilst maintaining cell viability (C). N=3. Mean ± SEM. Two-way ANOVA followed by a Dunnett’s post hoc test. On graph B statistical tests shown only for paclitaxel 1000 nM group (all paclitaxel doses were significant in comparison to the no insult group). *p<0.05, **p<0.01, ***p<0.001 and ****p<0.001.
To establish the model, it was important to ensure the cells had degenerated neurites but were still viable following insult. We classified degeneration as when the neurites have deteriorated, exhibiting beading and interrupted morphology, but the cell body was still visibly viable. We observed the cells every hour over a 24-hour period post-insult and established the optimal insult duration of 2-4 hours in this model, rather than the 24 hours which had been documented previously [35,58] (Figure 4).
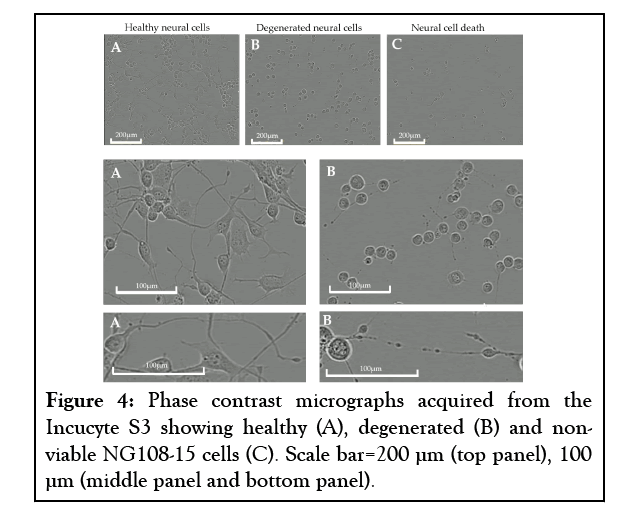
Figure 4: Phase contrast micrographs acquired from the Incucyte S3 showing healthy (A), degenerated (B) and nonviable NG108-15 cells (C). Scale bar=200 µm (top panel), 100 µm (middle panel and bottom panel).
Regenerative effect with a combination of vitamins B1, B6 and B12 in monolayer degenerated NG108-15 cells
After 4 hours the insult was removed and the cells were treated with or without a combination of vitamins B1, B6 and B12. After 24 hours the insulted cells treated with vitamins B1, B6 and B12 demonstrated a significantly higher mean neurite length, increased by 75.7% (p=0.0013) in comparison to the insulted cells that received no vitamins B1, B6 and B12 (Figure 5A). The cells treated with vitamins B1, B6 and B12 also demonstrated a longest neurite length of ~550 μm whereas the longest neurite length seen with no vitamin B treatment was ~290 μm (Figures 5B and 5C).
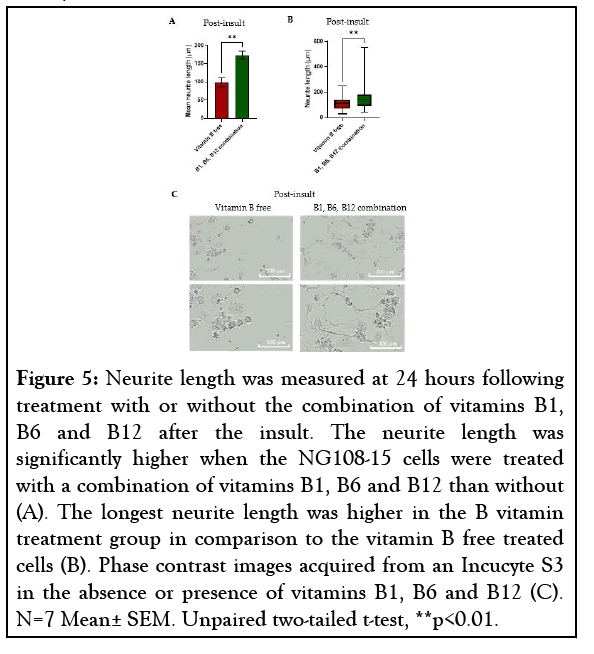
Figure 5: Neurite length was measured at 24 hours following treatment with or without the combination of vitamins B1, B6 and B12 after the insult. The neurite length was significantly higher when the NG108-15 cells were treated with a combination of vitamins B1, B6 and B12 than without (A). The longest neurite length was higher in the B vitamin treatment group in comparison to the vitamin B free treated cells (B). Phase contrast images acquired from an Incucyte S3 in the absence or presence of vitamins B1, B6 and B12 (C). N=7 Mean± SEM. Unpaired two-tailed t-test, **p<0.01.
Regenerative effect of vitamins B1, B6 and B12 in 3D co-culture of degenerated neural cells
To greater mimic a native nerve environment, our previous 3D co-culture engineered neural tissue model, consisting of a stabilized anisotropic collagen hydrogel containing aligned Schwann cells with NG108-15 cells seeded on the surface [25] was insulted with 10 µM H2O2, and subsequently treated with or without a combination of vitamins B1, B6 and B12. Upon 2-hour insult with H2O2, the NG108-15 neurite extension was reduced, indicating degeneration.
Adhesion of NG108-15 cells to the anisotropic Schwann cell collagen hydrogel was also reduced upon insult. This loss of NG108-15 cells was further amplified with lack of post-insult treatment. Treatment with vitamin B free medium following insult resulted in a 61.3% decrease in neurite length in comparison to the no insult control (19.55 ± 1.23 µm and 50.58 ± 1.10 µm (mean ± SEM)). Mean neurite extension 24 hours postinsult was significantly higher in the vitamin B combinationtreated cells, showing a 125.1% increase (Figure 6A), with the neurite extension almost returning to the neurite length seen when no insult was used (Figure 6A), (44.02 ± 7.65 µm and 50.58 ± 1.10 µm (mean ± SEM) respectively).
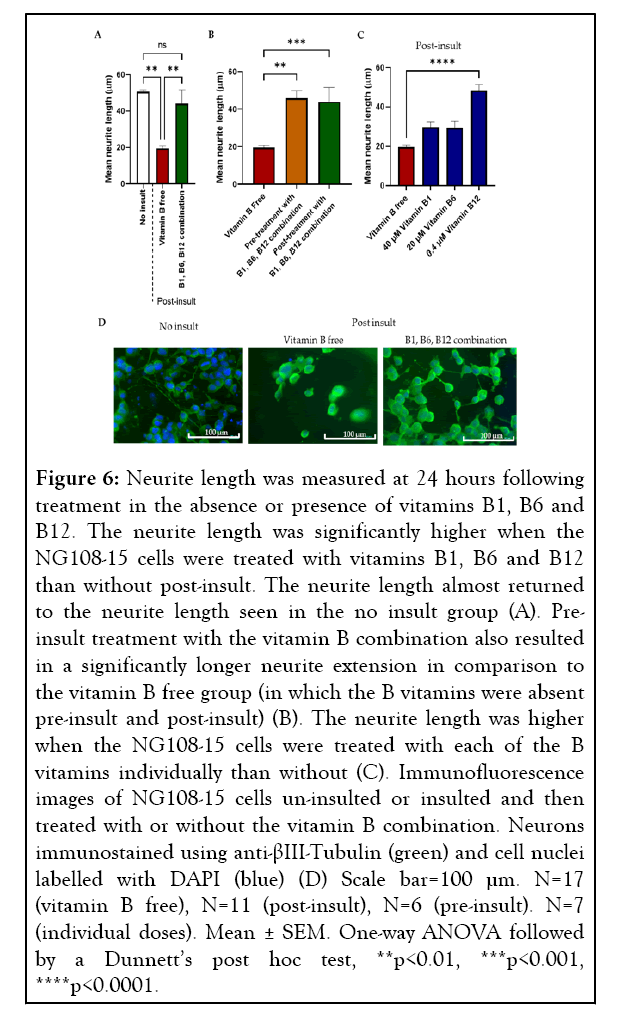
Figure 6: Neurite length was measured at 24 hours following treatment in the absence or presence of vitamins B1, B6 and B12. The neurite length was significantly higher when the NG108-15 cells were treated with vitamins B1, B6 and B12 than without post-insult. The neurite length almost returned to the neurite length seen in the no insult group (A). Preinsult treatment with the vitamin B combination also resulted in a significantly longer neurite extension in comparison to the vitamin B free group (in which the B vitamins were absent pre-insult and post-insult) (B). The neurite length was higher when the NG108-15 cells were treated with each of the B vitamins individually than without (C). Immunofluorescence images of NG108-15 cells un-insulted or insulted and then treated with or without the vitamin B combination. Neurons immunostained using anti-βIII-Tubulin (green) and cell nuclei labelled with DAPI (blue) (D) Scale bar=100 µm. N=17 (vitamin B free), N=11 (post-insult), N=6 (pre-insult). N=7 (individual doses). Mean ± SEM. One-way ANOVA followed by a Dunnett’s post hoc test, **p<0.01, ***p<0.001, ****p<0.0001.
As post-insult treatment with a combination of vitamins B1, B6 and B12 had shown to significantly promote neurite regeneration, we wanted to determine whether treatment preinsult had a similar effect. The neural cells were pre-treated with or without the vitamin B combination before the insult and both groups treated with vitamin B free medium following insult. An improvement in neurite extension was seen when the NG108-15 cells were treated with vitamins B1, B6, B12 before insult with a 134.7% increase (Figure 6B) compared to having none of these B vitamins added.
A similar magnitude of neurite extension increase was seen whether the cells were treated pre or post-insult with vitamins B1, B6 and B12 with a mean neurite length of 45.89 ± 3.88 μm and 44.02 ± 7.65 μm respectively in comparison to no vitamin B treatment 19.55 ± 1.23 μm (mean ± SEM). Following a significant effect seen using a combination of vitamins B1, B6 and B12, it was important to determine whether they have an individual effect on neurite extension. The same 3D co-culture system was used to screen the effect of 40 μM vitamin B1, 20 μM vitamin B6, and 0.4 μM vitamin B12 in isolation. A trend of all three B vitamins individually increasing neurite extension was seen, 0.4 μM vitamin B12 gave a 147.1% increase in neurite extension, 20 μM vitamin B6 a 50.0% increase and 40 μM vitamin B1 a 51.6% increase, however, a significance difference was only seen with vitamin B12 (Figure 6C).
To investigate the effect of neurotropic B vitamins (vitamins B1, B6 and B12) on neurite regeneration, an in-vitro model capable of neurite outgrowth after a neurodegenerative event must first be developed. Here we adapted a 3D-engineered co-culture system to study compounds that modulate neurite degeneration [25]. Of the four insults investigated, paclitaxel, suramin and H2O2 were capable of inducing degeneration of NG108-15 cell neurites without impacting cell viability. Suramin was eliminated as it promoted neurite growth at some doses rather than inducing neurodegeneration. The effect of suramin is debated in the literature with some studies claiming suramin to be neurotoxic whereas others demonstrated beneficial effects on nerve regeneration [59-61]. At the concentrations used here, paclitaxel caused neurite length to decrease over time, with no clear dose-response relationship, making it difficult to optimize as an insult within a standardized reproducible model. H2O2 was selected as the most appropriate insult for use in the model because it exhibited a dose-response relationship, with 5-10 µM H2O2 inducing degeneration of neurites. Neurite growth was restored following H2O2 insult removal but remained shorter than the no insult control. H2O2 is believed to induce neurite degeneration through oxidative stress, as a result of reactive oxygen species [58,62,63]. The metabolic pathways impaired in diabetic peripheral neuropathy are said to converge to increase oxidative stress, proposing oxidative stress as one of the initiators of diabetic peripheral neuropathy [64,65]. The choice of H2O2 in this model is therefore relevant since the intended use is to screen neuropathy treatments, particularly for diabetic peripheral neuropathy given it is the most common [5].
Once the insult type and duration had been optimized in the monolayer set-up, the model was advanced to 3D to improve its representativeness of native peripheral neuropathy by the addition of an anisotropic collagen matrix containing aligned Schwann cells [36]. The extracellular matrix in the peripheral nerve consists mainly of collagen which plays a key role in the development and maintenance of healthy nerves [66]. Therefore the incorporation of collagen into our model provided a tissuelike structure, with a biophysical and biochemical environment that resembles nerve tissue [67]. Embedding Schwann cells within the collagen matrix further improved the representativeness of our in-vitro model. Schwann cells provide support to neurons during damage and repair, meriting their incorporation to recreate this cell-to-cell interaction within the model [68]. Cell choice needs to be considered carefully when developing a model of nervous system disease or injury. Primary cells are sometimes favored, particularly for the neuronal component, but immortalized cell lines can be more readily available, easily expanded, standardized and more reproducible [25]. The choice of cells depends largely on the specific biological effect that is under investigation. In our previous study, neurite regeneration in-vitro was comparable when using either cell lines or primary neurons in 3D co-culture, indicating that the combination of NG108-15 and SCL4.1/F7 cell lines is suitable for modelling neurite responses in this model [25]. The decreased neurite length observed upon insulting the 3D coculture for 2 hours with H2O2 gave the desired neurodegenerative effect, as optimized in the monolayer version of the model. After model verification, we used the 3D coculture to investigate the effect of pre-insult and post-insult vitamin B1, B6 and B12 combination treatment. As far as we are aware, this is the first time the neuroregenerative capacity of neurotropic B vitamins B1, B6, B12 has been shown in a 3D co-culture neuropathy model.
In both our monolayer and 3D co-culture models, post-insult vitamin B1, B6 and B12 combination treatment showed neurite extension to increase by 75.7% and 125.1%, respectively, which is consistent with the improvement of peripheral neuropathy signs and symptoms seen with the administration of such a B vitamin combination (B1, B6 and B12) in animal [69-71] and clinical studies [43,44,47-50]. For example, in rats with diabetic or acrylamide-induced neuropathy, this B vitamin combination improved nerve conduction velocity [69,70] and also ameliorated tactile allodynia and formalin-evoked hyperalgesia [69]. Beyond that, vitamin B1, B6 and B12 combination treatment showed a clinically relevant improvement in sensory symptoms [43,44,47], nerve conduction velocity, and vibration perception threshold in humans suffering from peripheral neuropathy [48-50].
In addition, not only post-treatment but also pre-treatment with B vitamins showed neurite extension to reach a similar level compared to the no treatment control. These results indicate that neurotropic B vitamins may have a neuroprotective effect in addition to a neuroregenerative effect. However, further research is needed to study the mechanisms underpinning each of these effects. Despite there being evidence that vitamins B1, B6 and B12 act synergistically [41], it is also important to screen the effect of each B vitamin in isolation. It is commonly accepted that the three vitamins possess distinctive but important functions in the nervous system, with vitamin B1 being mainly involved in energy metabolism and antioxidation, B6 in neurotransmitter synthesis [73,74], and B12 in myelin synthesis [41].
In our study, administration of each B vitamin individually as well as the combination increased NG108-15 neurite extension after insult compared with the no-treatment control, but to varying degrees. Vitamin B12 was the most effective of the individual B vitamins tested here, increasing neurite length in a manner consistent with a previous study reporting neurite growth in DRG and cerebellar granule neurons and increased myelinated axons following a rat sciatic nerve crush injury [75]. Vitamin B1 has been shown previously to contribute to maintaining nerve cell function, similarly to vitamin B6 [41,42,76-78]. It is undoubtedly not yet fully understood how the neurotropic B vitamins protect against or support recovery from peripheral neuropathy [42]; advancing the knowledge of the mechanistic properties of these B vitamins will help progress our understanding of how they benefit nerve regeneration.
In conclusion, we established a model of neuropathy by demonstrating neurodegenerative changes in neural cells through the retraction and degeneration of previously healthy neurites in response to relevant insults. The utilization of this model allowed us to demonstrate the positive effects of neurotropic vitamins B1, B6 and B12 on neurite regeneration in a monolayer and, for the first time, in a 3D co-culture system. These beneficial effects have been observed with both post-insult use of the individual B vitamins, and pre- and post-insult use of the combination, suggesting neuroregenerative effects. To gain further insight into the mechanistic properties of vitamins B1, B6 and B12, our future work will involve exploring their beneficial effects on particular cell types and signalling pathways.
Special thanks go to Despoina Eleftheriadou.
This research was funded by P&G Health Germany GmbH. P&G Health was involved in the interpretation of data; in the writing of the manuscript and in the decision to publish the results.
With competing interests Patrizia Bohnhorst who is employee of P&G Health Germany GmbH. The authors from UCL Centre for Nerve Engineering provided paid consultancy to P&G Health Germany GmbH.
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
[Cross Ref] [Google Scholar] [PubMed]
Citation: Smith PO, Trueman RP, Powell R, Gregory H, Phillips JB, Bohnhorst P, et al. (2023) Exploring the Effect of Vitamins B1, B6 and B12 on Neurite Regeneration using a 3D Co-Culture Model of Neurodegeneration. Int J Phys Med Rehabil. 11:667.
Received: 13-Feb-2023, Manuscript No. JPMR-23-21794; Editor assigned: 15-Feb-2023, Pre QC No. JPMR-23-21794 (PQ); Reviewed: 01-Mar-2023, QC No. JPMR-23-21794; Revised: 08-Mar-2023, Manuscript No. JPMR-23-21794 (R); Published: 15-Mar-2023 , DOI: 10.35248/2329-9096.23.11.667
Copyright: © 2023 Smith PO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.