Research Article - (2022)Volume 8, Issue 4
Evaluation of In Silico and In Vitro Inflammatory and Biofilm Targets Inhibition by the Polyphenols of Prunus persica L. Spp; Functional Food Based Approach for the Illnesses Associated with Inflammatory and Microbial
Abdul Rafey1*, Tooba Khalid1, Madiha Ahmad2, Fakhar ul Mahmood2, Farhat Shaheen1, Abdul Haleem Shah3 and Samir Anis Ross4Abstract
Aims and objectives: Considering the increasing resistance of bacteria to antibiotics and the presence of antibacterial agents in plants being served as functional food choice for many ailments. Chronic diseases become the leading cause of more than 50% of deaths around the world. The real reason is inflammation linked to the microbial association as biofilm trend behind all chronic diseases like congestive heart failure, cognitive disorders, diabetes and associated comorbidities, hypertension. Bioactive compounds in functional foods are responsible for all these health benefits present in them. Research has suggested that fruits of Prunus persica L. rich in polyphenolic constituents as major bioactive compounds that possess remarkable antibacterial properties. The aim of this study was to evaluate the anti-biofilm and anti-inflammatory potential of the polyphenolic constituents of the fruits of Prunus persica L.
Methodology: During this project, both in vitro and in silico models were adopted to investigate the objectives of the study. Several fractions of the peel of Prunus persica were subjected to initial antimicrobial and anti-biofilm screening. Based on structural diversity, selected pure compounds were screened for putative binding sites and molecular docking studies followed by enzymatic analysis of 15 LOX inhibition and anti-biofilm potential.
Results and discussion: The negative binding energies and close proximity to residues in the binding pocket of selected targets including human α-soybean LOX (PDB ID 1IK3) and LasR (2UV0) were recorded, which indicated high affinity and tight binding capacity of gallic acid and Ferulic acid towards the active sites of LasR 2UV0 and 15-lipoxygenase. P-coumaric acid exhibited the highest inhibition of 15-lipoxygenase in vitro (70%, at 0.033 mM final concentration) and that is accompanied by ferulic acid (65%,) whereas in the biofilm inhibition assay, gallic acid was most active (IC50 0.05 mM), followed by Chlorogenic acid (IC50 0.07 mM), It was therefore concluded that gallic acid and Chlorogenic acid had the highest biofilm inhibitory activity, whereas ferulic acid and p-Coumaric acid were potent 15-lipoxygenase inhibitors with potentially anti-inflammatory properties.
Conclusion: By considering the results obtained and increasing resistance of bacteria to chemical antibiotics, it is suggested that several bacterial infections and associated inflammatory conditions may be treated using the above functional food based approach by employing the polyphenolic constituents of the Prunus persica L.
Keywords
Biofilm; 15 Lox; Functional foods; Transcriptional regulators; Molecular docking; HYDE assessment
Introduction
Functional foods are foods or dietary components that have the potential to provide health benefits beyond basic nutrition.A food can be made functional by using any technological or biotechnological means to increase the concentration of, add, remove, or modify a specific component, as well as improve its bioavailability, provided that component has been shown to have functional effect, introducing the conception of probiotics and prebiotics, which may affect microbial configuration and activities [1].
Prunus persica L. is a member of the Rosaceae family and is one of the most popular stone fruits for direct consumption as well as an interesting material for the food industry. P. persica (Rosaceae) is the second most widely cultivated fruit tree [2]. Because of its bioactive constituents (phenols and carotenoids), peach fruit is well-known for its nutritional value and therapeutic properties [3]. As a result, significant efforts have been directed toward estimating the bioactivity and functionality present in various peach varieties in order to evaluate the potential of peach fruits. Several studies examined the bioactive constituents of P. persica extracted with various solvents. Methanol was the most commonly used of them all [4].
Phenolic compounds are a diverse and large class of compounds. The nature of those compounds varies from organ to organ within each plant species but remains constant. It is well known that traditional fruits and vegetables have numerous health benefits [5]. The beneficial effects of these natural products are attributed to their bioactive compounds, primarily phenolic compounds and glycosides [6-8]. It is well understood that phenolic compounds have antioxidant properties and help to prevent the oxidation of low density lipoprotein cholesterol (LDL-C) [9]. Fruits' healthpromoting properties are due to the presence of some secondary metabolites, such as phenols, which have received a lot of attention due to their disease-fighting potential. Furthermore Microbial pathogenesis is thought to be the link between disease onset and aging-related inflammation [10].
Biofilms are accumulation of microorganisms attached to the surface and embedded in a polymeric substance extracellular, with cell masses ranging from 108 to 1011 cells g-1 [11,12]. Biofilms are the most common type of successful and distinct life on the planet. Biofilms are actually adaptations of various systems. Biofilm producing microbes can infiltrate higher organisms, including humans and may be linked to obstinate infections in humans, plants, and animals [13,14]. One of the most dangerous implications involved in many localised type infections of chronic nature that offer resistance to conventional antibiotics is the microbial biofilm [15]. Microorganisms in biofilms adapt to available conventional therapeutic options by modifying molecular or metabolic pathways, resulting in resistance [16]. With greater understanding of the ingredients contained in stone fruit kernels and their health-promoting potential, there is growing interest in their use for human consumption, particularly to enrich man's diet with compounds beneficial in the prevention of chronic inflammation and a variety of diseases [17-19].
According to a review of the literature, Polyphenolic compounds isolated from Prunus persica L. plants have a wide range of activities, including anti-leishmanial, anti-inflammatory, cytotoxic, anticancer, antibacterial, and antiviral properties [20,21] . It has been suggested by several investigations [22]. Immunological changes (including chemokine and cytokine levels) caused by inflammation may increase the pathogenicity of microbial infections, and a crossdependent relationship has been discovered. Therefore, we aimed to use in vitro and in silico techniques to investigate the antibiofilm and anti-inflammatory activities (15 LOX inhibition) of the Polyphenolic compounds from the peel of Prunus persica L.
Materials and Methods
Bacterial strains, growth media and chemicals
The commercial strains used during investigation included Staphylococcus aureus (ATCC 33862) and Pseudomonas aeruginosa (ATCC 15442). The bacterial growth media used included, Tryptic Soya Broth (TSB), nutrient agar (Hi Media, India) Luria-Bertani Broth (LB) (Oxoid). The standard compounds were purchased commercially -the Polyphenolic compounds i.e., Gallic acid (A), Protocatechuic acid (B), Chlorogenic acid (C), p-Coumaric acid (D) and Ferulic acid (E) [23]. were purchased commercially, including Gallic acid (Fluka Honeywell, Seelze, Germany), quercetin (Sigma Aldrich, St. Louis, MO, USA), Protocatachuic acid (Fluka Honeywell, Seelze, Germany), ciprofloxacin (Sigma Aldrich, St. Louis, MO, USA) ferulic acid (Sigma Aldrich, Steinheim, Germany), chlrogenic acid (Sigma Aldrich, Steinheim, Germany),P-coumaric acid (Fluka Honeywell, Seelze, Germany). All the chemicals purchased were of analytical grade.
The plant material: Plant materials were purchased from the local market of Islamabad Pakistan and were authenticated in the department of Botany Quaid e Azam University Islamabad. The Peel extract was prepared by cold maceration (90% methanol) for 10 days (repeated three times) and further liquid-liquid fractionation was accomplished using a rotary evaporator (40°C). The dried plant material was stored at -4°C until further usage. The schematic fractionation scheme is attached with supplementary material.
Phytochemical analysis: The phytochemical screening for different chemical classes was performed according the published reference.
Thin layer chromatography: The TLC was accomplished by using analytical TLC F254 plates [(20 20 cm) (Merck)] for normal phase. Various solvents systems were tried for a complete separation of bands. For obsrvation, UV lamp (360nm) and spraying with reagent p-anisaldehyde, reagent was employed.
Chromatographic analysis/HPLC profiling: A detailed HPLC analysis of total extract was performed using Agilent® 1200 series sys-tem (HPLC-DAD, Agilent Technologies and Santa Clara, CA, USA). The samples (10 μL) were injected at a flow rate of 1 mL/min with acetonitrile: water (0.1% formic acid) gradient (5% Acetonitrile to 100% in 40 min). Samples were prepared in a concentration range from 1 to 10 mg/mL in methanol. A Phenomenex Luna C18 column (silica-based, 250 × 4.6 mm, 5 μm) (Phenomenex, Torrence, CA, USA) was used.
Molecular docking
Ligand and protein structure preparation: For docking studies, the X-ray crystallographic structures of transcriptional regulators LasR (2UV0), (Yu et al.) and soybean LOX (PDB ID 1IK3) were extracted from the RCSB Protein Data Bank [24,25]. The 3D structures of the test compounds were prepared by MOE builder tool (MOE 2014) and energy minimization was done at MMFF94x force field (Labute). Afterwards the energy minimization and protonation of downloaded proteins was carried out by MOE (2014). A total number of 45 poses were generated and grouped according to their RMSD and best docked molecules [ΔG] were analyzed using Ligplot+Accelrys DS Visualizer 2.0 and PYMOL.
In vitro assays
Inhibition of 15-Lipoxygenase: The 15-lipoxygenase (15-LOX) assay was accomplished using the method developed by Malterud, et al. with a slight modification [26]. The % inhibition of enzyme activity was calculated as:
%Inhibition=(1-Absorbance of sample/Absorbance of control × 100)
Determination of MIC and MBC (minimum inhibitory and bactericidal concentrations): The isolated compounds were tested for antimicrobial activity using a slightly modified version of the modified method [27]. In the MIC assay, 50 L of the overnightgrown bacterial strain Pseudomonas auruginosa (ATCC 15442) (1.5107 CFU/ mL) and Staphylococcus aureus (ATCC 33862) were loaded into the 96 microwell plates, followed by 50 L of test sample (various dilutions). For 24 hours, the plates were incubated at 37 °C. The following day, 40 L of resazurin solution (0.015%) was added to each well, followed by another 60 minutes of incubation at 37 °C. A 96-microplate reader was used to take colorimetric readings (Hippo MPP-96, Biosan). For MBC values, bacterial suspensions (10 µL) from the MIC micro wells were transferred to already prepared agar plates (Muller Hinton) and incubated for 24 hours. Following that, bacterial growth on agar plates was monitored. All samples were loaded in triplicate. Ciprofloxacin was used as a positive control.
Anti-biofilm activity: The biofilm formation assay was carried out in 12-well polystyrene plates according to a slightly modified protocol [28,29]. In brief, the bacterial strain (P. aeruginosa ATCC 15442) was inoculated in 280 L of TSB medium with an initial turbidity of 0.5 at 600 nm (0.5 McFarland) and then incubated for 24 hours to produce biofilm. Following that, 100 µL of test compound (0.01-3 mg/mL) was added to the bacterial culture, which was then incubated at 37°C for another 24 hours. At 592 nm, cell growth in the plates was measured. Crystal violet was used to stain the biofilms in the 12-well plates for quantification. After that, 95% ethanol was added to the stained cells, and absorbance at 592 nm was measured to quantify total biofilm formation. The % inhibition was calculated using following formula,
% inhibition=(1-Abs of sample/Abs of control x 100)
Results
Biofilm molecular docking
Molecular docking studies of test compounds were carried out in the active pocket of transcriptional regulators LasR (2UV0). The transcriptional regulator LasR is present in homo-tetramer form (chain E, F, G and H), and for docking chain E was selected based on earlier reports. The binding interactions among the amino acid residues and test compounds inside the active site of transcriptional regulator LasR, 2UV0 were visualized by Discovery Studio. The results are presented in the form of 3D and 2D molecular interactions. The amino acids involved in the active pocket are Glu112, Arg163, Gly158,Gly138,Trp111, Pro 149, Phe 7, Gly 6,Ser 13, Glu 11, Gly 15. The molecular docking studies demonstrated that the compounds fit in near vicinity to the active pocket of the regulator; however, notable interactions are shown by the test compounds with amino acids His 63, Trp 60, Glu 104, Pro 57, Lys 34, Gly 109 Ala 107, Ala 108,Glu11, Ser161,Leu 165, Gly15,Ser 14,Trp19, Ser13, Leu 10, Trp 19, Gly 164, Ser161, Leu165,Ser14,Ser13, Leu10. The binding affinity and the means of interaction of the atoms in each molecule is shown in the 3D binding modes (Figures 1-3). The cognate ligand, N-3- oxo-dodecanoyl-L-homoserine lactone, after docking in the protein pocket, showed the same interactions as presented earlier [30]. The transcriptional regulator PqsE is present in a homo dimer form (chain A and B), and for docking chain A was selected based on earlier reports. All the docked molecules occupied well the binding pocket site and presented several important interactions. The 3D and 2D interaction diagrams of all the compounds are given in Figures 4-7.
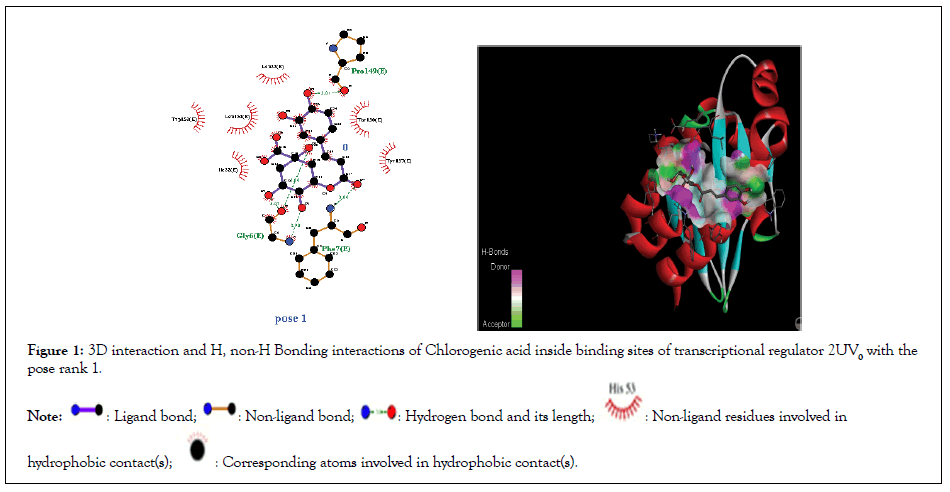
Figure 1:3D interaction and H, non-H Bonding interactions of Chlorogenic acid inside binding sites of transcriptional regulator 2UV0 with the pose rank 1.
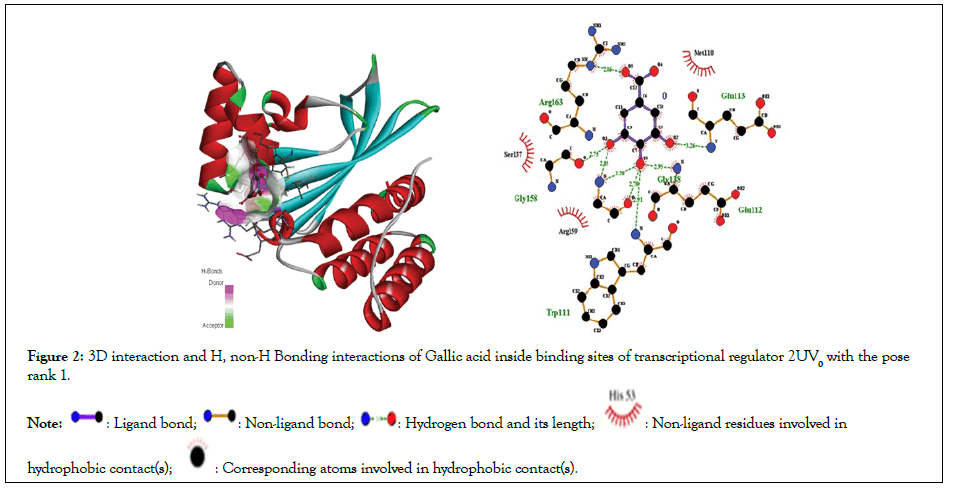
Figure 2:3D interaction and H, non-H Bonding interactions of Gallic acid inside binding sites of transcriptional regulator 2UV0 with the pose rank 1.
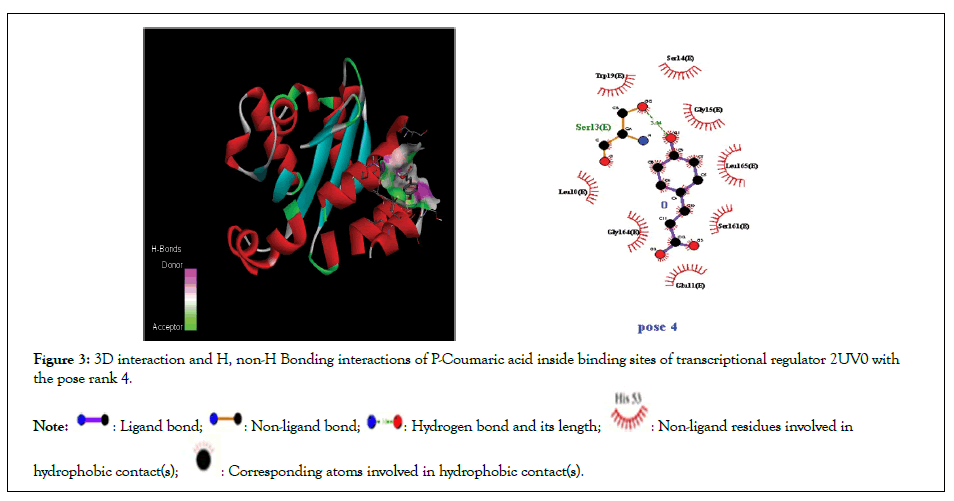
Figure 3: 3D interaction and H, non-H Bonding interactions of P-Coumaric acid inside binding sites of transcriptional regulator 2UV0 with the pose rank 4.
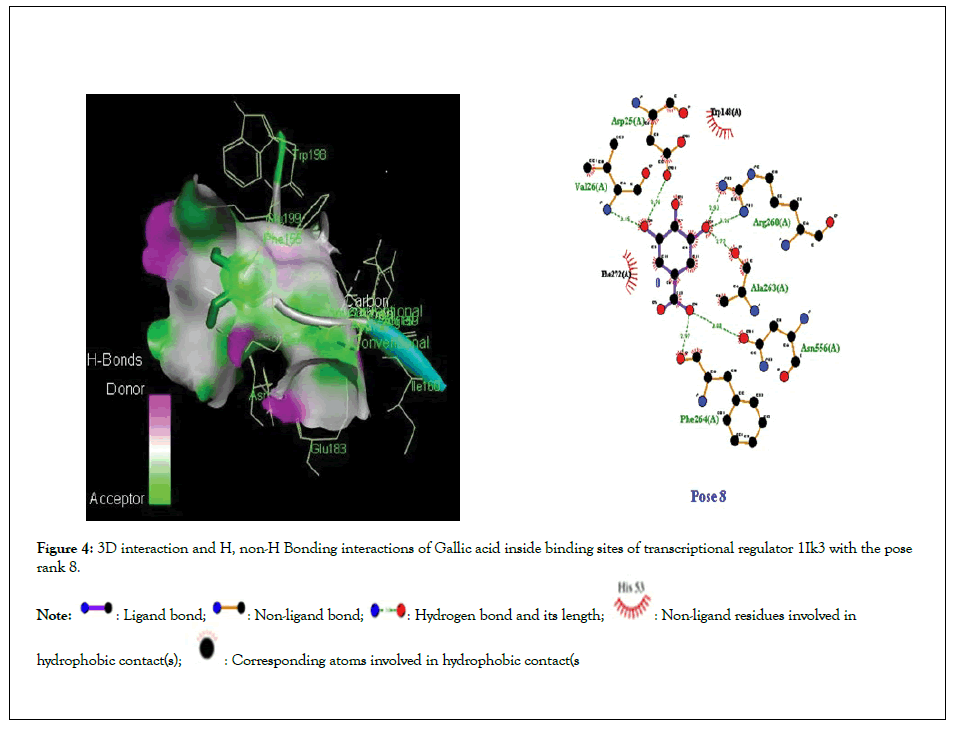
Figure 4:3D interaction and H, non-H Bonding interactions of Gallic acid inside binding sites of transcriptional regulator 1Ik3 with the pose rank 8.
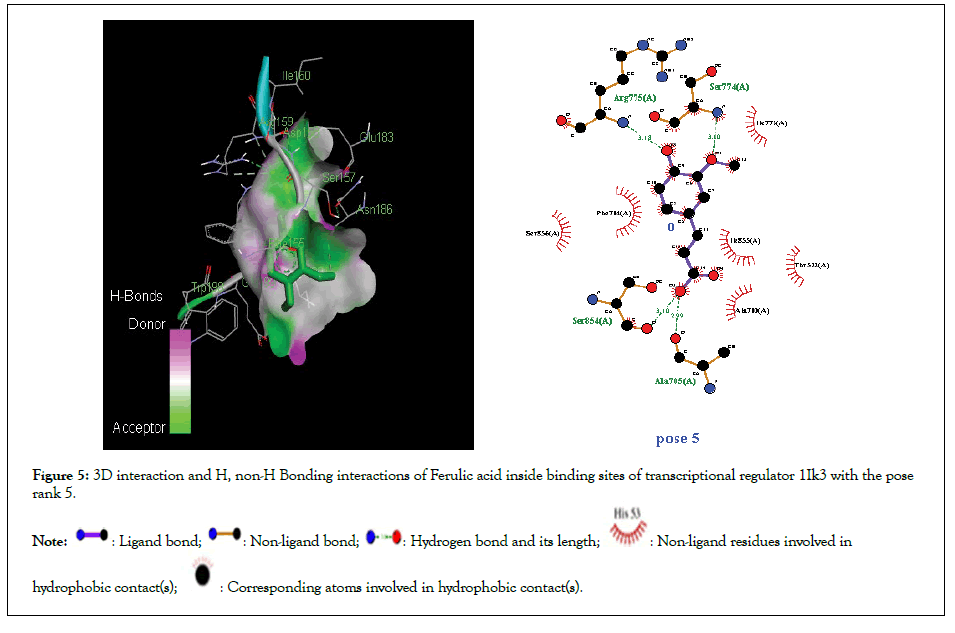
Figure 5:3D interaction and H, non-H Bonding interactions of Ferulic acid inside binding sites of transcriptional regulator 1Ik3 with the pose rank 5.
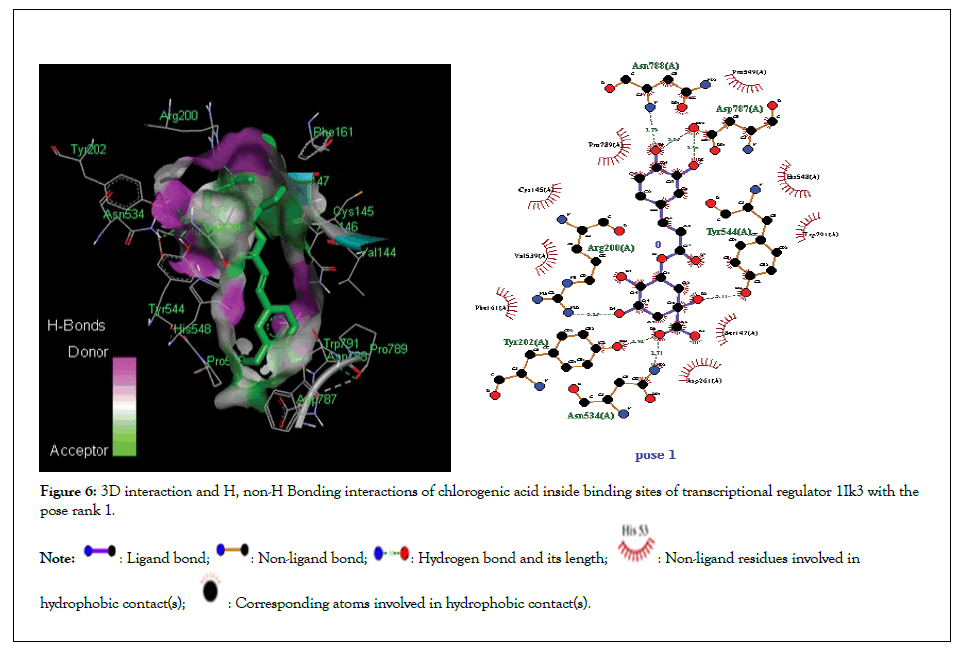
Figure 6: 3D interaction and H, non-H Bonding interactions of chlorogenic acid inside binding sites of transcriptional regulator 1Ik3 with the pose rank 1.
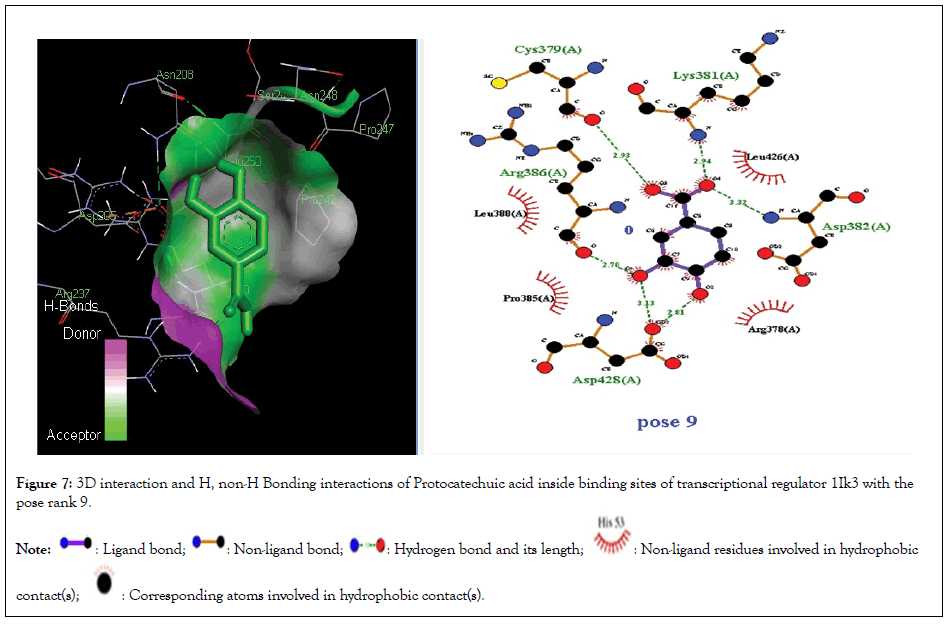
Figure 7:3D interaction and H, non-H Bonding interactions of Protocatechuic acid inside binding sites of transcriptional regulator 1Ik3 with the pose rank 9.
15-LOX docking
Molecular docking studies of test compounds was carried out for the possible inhibition of soybean 15-LOX PDB 1IK3 in Table 1. Along with the FlexX score of the top ranking pose, HYDE assessment provided the binding free energies of all the test compounds within the active pockets of receptors. Molecular docking studies of all test compounds were carried out inside the soybean 15-LOX PDB 1IK3. The results are depicted in Figures 4-7 and supplementary (Figures 4-7). Among all compounds, the most notable interaction was seen with regard to compound 1 in Figure 4 that was inside the binding pocket of 1IK3 encompassing hydrogen bonding with residues Phe 264,Asn556, Ala263,Arg 260,Asp25, Val 26. The common hydrogen bonding interaction with residue Arg 200 is shown by the ligand of 1IK3, Chlorogenic acid and p-Coumaric acid. The putative interactive mode of compound 1 is shown in Figure 4.To the left side, the 3D interactions are shown, whereas on the right side, a cartoon view of the docked compound inside 1IK3 is displayed. Similarly, compound 3 and 5 appeared to be potent inhibitor of 15 LOX. It was found to bind inside the active site of 1lK3 with a binding free energy of -9.2 and -5.7 kJ mol -1 with the best pose rank of 1 and 5 respectively. On the other hand, compound 4 gave hydrogen bonding interactions with Arg 159, Arg 200, Asn 186, Ser 157. Based on interactions, important residues involved in the formation of hydrogen bonds and π-π interactions for all other compounds were Phe 264,Asn556, Ala263,Arg 260,Asp25, Val 26, Asp 428, Asp 382, Arg , Arg 159, Arg 200 , Asn 186, Ser 157. The facts describe the interactions of secluded compounds inside the soybean 15-LOX 1IK3 (Figure 8).
Figure 8: The structures of standard commercial compounds used, A: Gallic Acid; B: Procatechuic acid; C: Chlorogenic acid; D: P-coumaric acid; E: Ferulic acid.
HYDE assessment of compounds against all the targets: The HYDE affinity assessment was done for the first 30 top ranked docking conformations within the active sites of 1IK3 and 2UV0 and it helped in the selection of the correct binding mode. The binding free energy ΔG, FlexX docking score and the most favorable poses for all the compounds are given in Table 1. The compounds bind to the receptor with a very high binding affinity and give favorable contributions.
| Compound | Binding free energy ΔG (kJ mol‒1) | Pose rank | No of H bonds | H Bond interaction residues | Other interaction residues |
|---|---|---|---|---|---|
| Gallic acid | -5.8 | 1 | 5 | Glu112,Arg163,Gly158, Gly138,Trp111 | Met110,Arg159,Ser137,Ile139 |
| Protocatechuic acid | -6 | 8 | 0 | - | His 63, Trp 60, Glu 104, Pro 57, Lys 34, Gly 109 Ala 107, Ala 108 |
| Chlorogenic acid | -6.1 | 1 | 3 | Pro 149, Phe 7, Gly 6 | Ile22, Typ 157, Trp 152, Leu 33, Trp 153 |
| p-Coumaric acid | -5.3 | 8 | 1 | Ser 13 | Glu11, Ser161,Leu 165, Gly15,Ser 14,Trp19, Ser13, Leu 10, |
| Ferulic acid | -5.6 | 6 | 2 | Glu 11, Gly 15 | Trp 19, Gly 164, Ser161, Leu165,Ser14,Ser13, Leu10, |
| Quercetin | -8.8 | 5 | 7 | Tyr46,Asp65,Arg61,Trp60 Ser129,Thr75,Thr115 |
Trp88,Ala127,Leu36,Val76, Ile52,Gly38,Ala50,Tyr64, Leu110,Tyr56, Asp73 |
| 1IK3 | |||||
| Gallic acid | -5.6 | 8 | 6 | Phe 264,Asn556, Ala263,Arg 260,Asp25, Val 26 | Trp 148, Phe 272 |
| Protocatechuic acid | -5.3 | 9 | 5 | Asp 428, Asp 382, Arg 386, Cys 379, Lys 381 | Arg 378, Pro 385, Leu 380, Leu 426 |
| Chlorogenic acid | -9.2 | 1 | 6 | Asn 534,Tyr 202, Arg 200, Tyr 544, Asp 787, Asn 788 | Asp 261, Ser 147, Trp 791, Phel 61, Val 539, Pro 789 |
| p-Coumaric acid | -6.1 | 1 | 4 | Arg 159, Arg 200 , Asn 186, Ser 157 | Ile201, Glu 199, Phel 155 |
| Ferulic acid | -5.7 | 5 | 5 | Ala 705, Ser 706, Ile 772, Ser 774, Arg 775 | Leu 708, Thr 522, Ile 855, Ala 710,Ser 856, Ser 854, Pro 852 |
Table 1: Docking score, H and and non H-bonding interactions of tested compounds.
In vitro analysis: The isolated fractions of the extracts of peel of Prunus persica L were initially analyzed for determination of MIC and MBC against biofilm producer strain Pseudomonas aeruginosa (ATCC 15442). Among all fractions, methanol extract exhibited significant activity (MIC 0.018 mg/mL, MBC 0.018 mg/mL) and followed by Aqueous and chloroform fractions (MIC 0.29 mg/mL, MBC 0.29 mg/mL) Table 2, whereas other fractions produced no significant inhibition of the strain (MIC>3 mg/mL). The fractions were also screened for Staphylococcus aureus (ATCC 33862) as well since it’s the most common pathogenic bacteria involved in many diseases and inflammatory conditions and the results were found to be significant. Among all fractions, methanolic fraction followed by chloroform and Aqueous were able to show remarkable biofilm as well as 15 LOX inhibition (Tables 3 and 4). Pure compounds were further tested for the antibiofilm potential against the biofilm producing strain P. aeruginosa (ATCC 15442). Amongst all analyzed compounds, compound A displayed highest biofilm inhibition followed by 3 and 5 with the IC50 value of 0.05 , 0.07 and 0.28 respectively (Table 5).
| Extracts | Staphylococcus | Pseudomonas aeruginosa | ||
|---|---|---|---|---|
| MIC (mg/dl) | MBC (mg/dl) | MIC (mg/dl) | MBC (mg/dl) | |
| MeOH fract. | 0.03 | 0.03 | 0.18 | 0.18 |
| Ethyl acetate | 3.6 | ≥ 3.6 | 2.56 | ≥2.56 |
| Chloroform | 0.17 | ≥ 0.17 | 2.6 | 2.6 |
| N-Hexane | 4.2 | ≥ 4.2 | 3.1 | ≥3.1 |
| Aqueous | 0.48 | ≥ 0.48 | 0.29 | 0.29 |
| Standarda | 10 | 10 | 10 | 10 |
Note: a= Ciprofloxacin 10 µg /ml
Table 2: Antimicrobial properties of different fractions of extracts of peel of Prunus persica L.
| Extract part | Biofilm inhibition (IC50 mM) |
|---|---|
| MeOH fraction | 0.08 |
| Ethyl acetate | Not active |
| Chloroform | 0.09 |
| N-hexane | Not active |
| Aqueous | 0.27 |
| Standarda | 0.0231 |
Note: a=Quercetin (mM)
Table 3: Antibiofilm properties of different fractions of extracts of peel of Prunus persica L.
| Extract part | 15 LOX (%inhibition) |
|---|---|
| MeOH extract | 60 |
| Ethyl acetate | Not active |
| Chloroform | 36 |
| N-hexane | 28 |
| Aqueous | 65 |
| Standard | 66a |
Note: a=Quercetin (mM)
Table 4: Anti-inflammatory (15 Lox inhibition) by different fractions of the extracts.
| Compound | Biofilm inhibition (IC50 mg/ml) | 15-lox (% inhibition)* |
|---|---|---|
| 1 | 0.05 | 48 |
| 2 | Not active | 28 |
| 3 | 0.07 | 58 |
| 4 | Not active | 70 |
| 5 | 0.28 | 65 |
| Standard | 0.0221a | 182b |
Note: a-Ciprofloxacin µg/ml; b=Quercetin (mM), *(final concentration 0.033 mM final)
Table 5: Biofilm inhibition and 15 LOX inhibition exhibited by pure compounds.
On the other hand, in case of 15-LOX inhibition, compound D and E (0.033 mM final concentration) presented excellent inhibition (70% and 65%) whereas low inhibition was recorded for the other compounds in Table 5. The structural features may be involved in 15-LOX inhibition (Tables 6-8).
| Properties | Compounds | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| TPSA | 97.99 | 77.6 | 164.75 | 57.53 | 66.76 |
| Consensus Log Po/w | 0.21 | 0.66 | -0.38 | 1.26 | 1.36 |
| Absorption | |||||
| Log S (ESOL) (water solubility) | -1.64 | -1.86 | -1.62 | -2.02 | -2.11 |
| Pharmacokinetics | |||||
| GI absorption | High | High | Low | High | High |
| BBB permanent | No | No | No | Yes | Yes |
| P-gp substrate | No | No | No | No | No |
| CYP1A2 inhibitor | No | No | No | No | No |
| CYP2C19 inhibitor | No | No | No | No | No |
| CYP2C9 inhibitor | No | No | No | No | No |
| CYP3A4 inhibitor | Yes | Yes | No | No | No |
| Log Kp (skin permeation) | -6.84 | -6.42 | -8.76 | -6.26 | -6.41 |
| Drug likeness | |||||
| Lipinski | 0 | 0 | 1 | 0 | 0 |
| Bioavailability score | 0.56 | 0.56 | 0.11 | 0.85 | 0.85 |
| Toxicity | |||||
| Predicted LD50 | 2000 | 2000 | 5000 | 5000 | 1772 |
| Predicted toxicity class | 4 | 4 | 5 | 5 | 4 |
Table 6: ADMET analysis of the tested compounds.
| Extract part | Flavonoids | Terpenoids/Steroids | Alkaloids | Tannins | Saponins |
|---|---|---|---|---|---|
| MeOH fract. | +++ | ++ | -- | -- | -- |
| Ethyl acetate | +++ | ++ | -- | -- | -- |
| Chloroform | ++ | ++ | -- | -- | -- |
| N-Hexane | + | -- | -- | -- | -- |
| Aqueous | ++ | ++ | -- | + | -- |
Table 7: Quantitative phytochemical analysis of extracts of peel of Prunus persica L.
| Compound | Staphylococcus | Pseudomonas aeruginosa | ||
|---|---|---|---|---|
| MIC (mg/dl) | MBC (mg/dl) | MIC (mg/dl) | MBC (mg/dl) | |
| 1 | 0.28 | 0.28 | 0.25 | 0.25 |
| 2 | 4.2 | ≥ 4.2 | 3.56 | ≥ 3.56 |
| 3 | 0.076 | 0.012 | 0.068 | 0.068 |
| 4 | 5.2 | ≥ 5.2 | 3.1 | ≥ 3.1 |
| 5 | 1.87 | ≥ 1.87 | 0.099 | 0.099 |
| Ciprofloxacina | 8 | 8 | 8 | 8 |
Note: a=µ g/ml
Table 8: MIC and MBC values of pure compounds against staphylococcus aureus and Pseudomonas aeruginosa.
ADMET analysis: The ADMET properties of all compounds are shown in Table 2. The feature TPSA is related to the absorption properties of compounds, whereas Consensus Log Po/w is indicator for lipophilicity. It was evident that TPSA topological polar surface area was less than 100 that indicates good oral absorption or membrane permeability [31-35]. Likewise the compounds presented High to moderate lipophilicity [36,37]. The physicochemical and toxicity risk assessments were carried out using SWISS ADME explorer. The results are presented such as solubility, Topological Surface Area (TPSA), bioavailability, drug likeness and physicochemical characteristics. All compounds showed good solubility between –1.64 and –2.11. All five compounds were predicted to have strong drug likeness properties except compound C which showed only one violation of Lipinski rule of five. The toxicity risk assessment shows that compounds pose no risk as almost all were predicted to be occupying toxicity class five or nearest to five. Relatively all the compounds were able to possess good GIT absorption with no inhibition of the p-gp substrate. Among all, compounds D and E were predicted to cross the blood brain barrier. The detailed analysis of ADMET is shown in Table 6.
Discussion
The binding affinities of compounds A, C and E are in correlation with the experimental data as these compounds exhibited good inhibitory activity against biofilm and 15 LOX. The binding free energies and FlexX score showed that the compounds bind well into the active pocket with the good binding affinities and revealed favourable interactions. Interestingly there was very significant 15 LOX inhibition recorded for compound D (70%), unlike biofilm target, that are accordance with the best putative biding site and hydrogen interactions in active pocket site of transcriptional regulator 1IK3. ADMET analysis also revealed that compound crossing the blood brain barrier that may be involved in its highest percentage LOX inhibition with significant anti-inflammatory properties. Upon comparison of both in silico and in vitro analysis, it was evident that all compounds presented comparable binding energies to both targets, i.e., 1lK3 and 2UV0 (Tables 7 and 8). However, the binding affinities of few compounds were comparably as high as compound D and compound E, but these did not show activity during in vitro analysis. It is known that molecular docking analysis relies on computational screening and the results may [38,39]. In this case, despite hydrogen bond interaction, it could be hypothesized that complexes of compounds A and C and E might be stabilized by other non-covalent forces such as hydrophobic interactions and thus showed more activity compared with other tested molecules.
Conclusion
It was detected that compounds A, C and E were appeared to possess biofilm inhibitory potential, whereas compound D is potent LOX inhibitor followed by compound E with antiinflammatory properties. Thus, the antimicrobial, antibiofilm and anti-inflammatory characteristics of the Prunus persica L may be related due to the presence of the Polyphenolic constituents.
Authors’ Contributions
AR, TK, and FS performed analysis and interpretation of enzyme inhibitory data. AHS proposed the project and participated in drafting. MA and AR performed molecular docking and formal analysis. TK and FS participated in drafting and some formal analysis. AR designed the method and the main project, SAR participated in drafting, edited the manuscript, and gave the final approval of the version to be submitted.
Conflict of Interest
The author declares no conflict of interest.
References
- Ziemer CJ, Gibson GR. An overview of probiotics, prebiotics and synbiotics in the functional food concept: perspectives and future strategies. Int Dairy J. 1998;8(5-6):473-479.
- Abidi W, Jiménez S, Moreno MÁ, Gogorcena Y. Evaluation of antioxidant compounds and total sugar content in a nectarine [Prunus persica (L.) Batsch] progeny. Int J Mol Sci. 2011;12(10):6919-6935.
- Cantin CM, Moreno MA, Gogorcena Y. Evaluation of the antioxidant capacity, phenolic compounds, and vitamin C content of different peach and nectarine [Prunus persica (L.) Batsch] breeding progenies. J Agricultural Food Chem. 2009;57(11):4586-4592.
- Legua P, Hernández F, Díaz‐Mula HM, Valero D, Serrano M. Quality, bioactive compounds, and antioxidant activity of new flat‐type peach and nectarine cultivars: a comparative study. J Food Sci. 2011;76(5):C729-C735.
- Schreiner M, Korn M, Stenger M, Holzgreve L, Altmann M. Current understanding and use of quality characteristics of horticulture products. Scientia Horticulturae. 2013;163:63-69.
- Gorinstein S, Martin‐Belloso O, Lojek A, Číž M, Soliva‐Fortuny R, Park YS, et al. Comparative content of some phytochemicals in Spanish apples, peaches and pears. J Sci Food Agri. 2002;82(10):1166-1170.
- Alothman M, Bhat R, Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009;115(3):785-788.
- Silva S, Gomes L, Leitao F, Coelho AV, Boas LV. Phenolic compounds and antioxidant activity of Olea europaea L. fruits and leaves. Food Sci Tech Int. 2006;12(5):385-395.
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2(5):270-278.
- Vert M, Li SM, Spenlehauer G, Guérin P. Bioresorbability and biocompatibility of aliphatic polyesters. J Mat Sci: Mater Med. 1992;3(6):432-446.
- Balzer E, Heine C, Jiang Q, Lee VM, Moss EG. LIN28 alters cell fate succession and acts independently of the let-7 microRNA during neurogliogenesis in vitro. Development. 2010;137(6):891-900.
- Reunanen J, Kainulainen V, Huuskonen L, Ottman N, Belzer C, Huhtinen H, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appli Environ Microbiol. 2015;81(11):3655-3662.
- Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, et al. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41(1):435-464.
- Anjum MW, Vermoortele F, Khan AL, Bueken B, De Vos DE, Vankelecom IF. Modulated UiO-66-based mixed-matrix membranes for CO2 separation. ACS Appl Mater Inter. 2015;7(45):25193-25201.
- Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14(9):563-575.
[Cross Ref][Google Scholar][All Versions][Pubmed]
- Bak I, Lekli I, Juhasz B, Varga E, Varga B, Gesztelyi R, et al. Isolation and analysis of bioactive constituents of sour cherry (Prunus cerasus) seed kernel: an emerging functional food. J Med Food. 2010;13(4):905-910.
- Elshamy AI, Abdallah HM, El Gendy AE, El-Kashak W, Muscatello B, De Leo M, Pistelli L. Evaluation of anti-inflammatory, antinociceptive, and antipyretic activities of Prunus persica var. nucipersica (nectarine) kernel. Planta Med. 2019;85(11/12):1016-1023.
- Cha BC, Lee EH. Antioxidant and antiinflammation activities of Prunus persica tree extracts. Korean J Med Crop Sci. 2004;12(4):289-294.
- Tomlinson MS, Lu K, Stewart JR, Marsit CJ, O’Shea TM, Fry RC. Microorganisms in the placenta: links to early-life inflammation and neurodevelopment in children. Clin Microbiol Rev. 2019;32(3):e00103-e00118.
- Loizzo MR, Pacetti D, Lucci P, Núñez O, Menichini F, Frega NG, et al. Prunus persica var. platycarpa (Tabacchiera Peach): bioactive compounds and antioxidant activity of pulp, peel and seed ethanolic extracts. Plant Foods for Hum Nutri. 2015;70(3):331-337.
- Yu S, Jensen V, Seeliger J, Feldmann I, Weber S, Schleicher E, et al. Structure elucidation and preliminary assessment of hydrolase activity of PqsE, the Pseudomonas quinolone signal (PQS) response protein. Biochem. 2009;48(43):10298-10307.
- Skrzypczak-Jankun E, Bross RA, Carroll RT, Dunham WR, Funk MO. Three-dimensional structure of a purple lipoxygenase. J Am Chem Soc. 2001;123(44):10814-10820.
- Malterud KE, Gjøen T, Rishovd AL. Procedure for assay of 15-lipoxygenase inhibition.
- Weseler AH, Geiss HK, Saller R, Reichling JJ. A novel colorimetric broth microdilution method to determine the minimum inhibitory concentration (MIC) of antibiotics and essential oils against Helicobacter pylori. Die Pharmazie Int J Pharma Sci. 2005;60(7):498-502.
- Bazargani MM, Rohloff J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control. 2016;61:156-164.
- Chaieb K, Kouidhi B, Hosawi SB, Baothman OA, Zamzami MA, Altayeb HN. Computational screening of natural compounds as putative quorum sensing inhibitors targeting drug resistance bacteria: Molecular docking and molecular dynamics simulations. Comp Biol Med. 2022;145:105517.
- Ertl P, Rohde B, Selzer P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J Med Chem. 2000;43(20):3714-3717.
- Qidwai T. QSAR modeling, docking and ADMET studies for exploration of potential anti-malarial compounds against Plasmodium falciparum. In silico pharmacol. 2017;5(1):1-3.
- Fonteh P, Elkhadir A, Omondi B, Guzei I, Darkwa J, Meyer D. Impedance technology reveals correlations between cytotoxicity and lipophilicity of mono and bimetallic phosphine complexes. Biomet. 2015;28(4):653-667.
- Chen CH. Handbook of pattern recognition and computer vision. World Sci. 2015.
- Chen L, Zhang J, Zhu Y, Zhang Y. Molecular interaction of inorganic mercury (II) with catalase: a spectroscopic study in combination with molecular docking. RSC advances. 2015;5(97):79874-79881.
- Chen G, Swem LR, Swem DL, Stauff DL, O'Loughlin CT, Jeffrey PD, et al. A strategy for antagonizing quorum sensing. Mol Cell. 2011;42(2):199-209.
- Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Revi Cancer. 2013;13(11):759-771.
- Labute P. Protonate 3D: assignment of macromolecular protonation state and geometry. Chem Comp Group Inc. 2007.
- MOE (2014) The Molecular Operating Environment, Version 2014.0901. Chem Comp Group.
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer's Dement. 2013;9(1):63-75.
- Ren L, Qin X, Cao X, Wang L, Bai F, Bai G, et al. Structural insight into substrate specificity of human intestinal maltase-glucoamylase. Protein Cell. 2011;2(10):827-836.
- Roberfroid MB. What is beneficial for health? The concept of functional food. Food and Chem Toxicol. 1999;37(9-10):1039-1041.
- Schneider N, André P, Könyves V, Bontemps S, Motte F, Federrath C, et al. What determines the density structure of molecular clouds? A case study of Orion B with Herschel. Astrophys J Letters. 2013;766(2):L17.
Author Info
Abdul Rafey1*, Tooba Khalid1, Madiha Ahmad2, Fakhar ul Mahmood2, Farhat Shaheen1, Abdul Haleem Shah3 and Samir Anis Ross42Department of Pharmacology, Gomal University, Dera Ismail Khan, Pakistan
3Department of Sciences, Gomal University, Dera Ismail Khan, Pakistan
4Department of Pharmacognosy, University of Mississippi, Mississippi, USA
Citation: Rafey A, Khalid T, Ahmad M, ul Mahmood F, Shaheen F, Shah AH, et al. (2022) Evaluation of In Silico and In Vitro Inflammatory and Biofilm Targets Inhibition by the Polyphenols of Prunus persica L. Spp; Functional Food Based Approach for the Illnesses Associated with Inflammatory and Microbial Pathways. Appli Microbiol Open Access. 8: 228.
Received: 10-May-2022, Manuscript No. AMOA-22-17409; Editor assigned: 13-May-2022, Pre QC No. AMOA-22-17409(PQ); Reviewed: 30-May-2022, QC No. AMOA-22-17409; Revised: 08-Jun-2022, Manuscript No. AMOA-22-17409(R); Published: 15-Jun-2022 , DOI: 10.35284/2471-9315.22.8.228
Copyright: © 2022 Rafey A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.