Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Review Article - (2024)Volume 15, Issue 2
After diabetic retinopathy, Retinal Vein Occlusion (RVO) is the second most common disease of the retinal vessels that reduces visual acuity. The degree of retinal damage and visual acuity reduction correlate positively with the size and location of the obstructed venous vessel supplying the retinal area and with the possibility of collateral circulation formation. Optical Coherence Tomography Angiography (OCT-A) is a modern, non-invasive method of assessing haemodynamics in the retinal and choroidal vessels. OCT-A is helpful in diagnosing RVO and assessing treatment effects. In this review, we present the changes in OCT-A examination of RVO patients before and after treatment with intravitreal aflibercept injections. Anti-Vascular Endothelial Growth Factor (Anti-VEGF) treatment in the RVO patients reduces the Non-Perfusion Area (NPA), improves retinal blood flow, reduces retinal ischemia and cystoid macular oedema and improves visual acuity.
Optical coherence tomography angiography; Retinal vein occlusion; Aflibercept; Macular oedema
VEGF: Vascular Endothelial Growth Factor; RVO: Retinal Vein Occlusion; CRVO: Central Retinal Vein Occlusion; BRVO: Branch Retinal Vein Occlusion; MO: Macular Oedema; OCT: Optical Coherence Tomography; CRT: Central Retinal Thickness; FA: Fluorescein Angiography; SCP: Superficial Capillary Plexuses; DCP: Deep Capillary Plexuses; NPA: Non-Perfusion Areas; VD: Vascular Density; CCFA: Choriocapillaris Flow Area; ORFA: Outer Retinal Flow Area
The three factors of Virchow’s triad (venous blood flow slowing, coagulation and fibrinolytic system disorders, impaired vascular endothelial function) are involved in vein occlusion development. Consequently, hydrostatic pressure in venous vessels and capillaries increases, inflammatory processes and vascular endothelial cell dysfunction develop and VEGF concentrations in the vitreous body increase due to hypoxia and ischemia [1]. Thrombus development in the vessel lumen is associated with platelet adhesion and subsequent aggregation at the damaged endothelium. The thrombus gradually builds up in the venous vessel lumen, narrowing it until the lumen is completely closed [2]. The degree of retinal damage and visual acuity reduction correlate positively with the size and location of the occluded venous vessel supplying a given area of the retina and with the possibility of collateral circulation formation. Retinal Vein Occlusion (RVO) occurs as Central or branch RVO (CRVO or BRVO, respectively). While stump occlusion occurs less frequently than branch occlusion of the central retinal vein, it has worse consequences and prognosis. CRVO affects the posterior retinal pole and might cause irreversible deterioration of visual acuity due to ischemia and subsequent Macular Oedema (MO). RVO is most frequent in older patients with systemic diseases. The factors that predispose towards blood clot formation include those that increase blood viscosity. These factors occur in many systemic diseases, such as Waldenstrom’s macroglobulinemia, malignant diseases of the haematopoietic system, sickle cell anemia, thrombophilia, diabetes, hyperlipidemia, cardiovascular diseases and obesity. The predisposing factors also include oral contraceptive use, smoking and dehydration. Furthermore, the local factors include glaucoma, orbital diseases, hyperopia [3]. In CRVO, venous occlusion may occur in the lamina cribrosa or the venous vessels within the optic nerve and BRVO in numerous arterial and venous junctions and compression sites [4].
Optical Coherence Tomography (OCT) enables the detailed assessment of the retinal structure and is currently the most frequently requested test for diagnosing macular diseases. In RVO, OCT imaging evaluates the Central Retinal Thickness (CRT), the presence of intra-retinal hypo reflective spaces corresponding to a cystic MO, the MO size and morphology, oedematous cyst arrangement and intra-retinal hyper-reflective foci (Figures 1a and 1b and 2a and 2b).
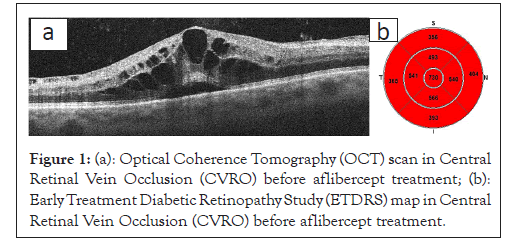
Figure 1: (a): Optical Coherence Tomography (OCT) scan in Central Retinal Vein Occlusion (CVRO) before aflibercept treatment; (b): Early Treatment Diabetic Retinopathy Study (ETDRS) map in Central Retinal Vein Occlusion (CVRO) before aflibercept treatment.
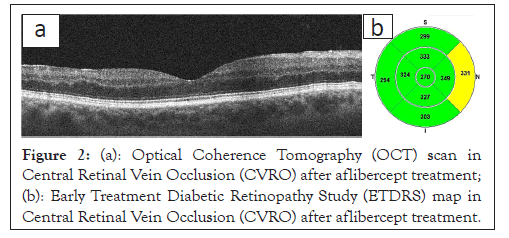
Figure 2: (a): Optical Coherence Tomography (OCT) scan in Central Retinal Vein Occlusion (CVRO) after aflibercept treatment; (b): Early Treatment Diabetic Retinopathy Study (ETDRS) map in Central Retinal Vein Occlusion (CVRO) after aflibercept treatment.
OCT-A is an OCT development and the latest non-invasive technique for imaging retinal and choroidal vessel structure and blood flow without requiring the use of contrast. Unlike Fluorescein Angiography (FA), which is a dynamic method, Anatomic OCT (OCT-A) is a static method that detects vessel flow at any given time and generates a constant image. OCT-A enables simultaneous assessment of the retinal structure and microcirculation function. Furthermore, its advantages include non-invasiveness and the ability to analyze individual vascular layers. RVO microvascular changes are visible in the Superficial Capillary Plexuses (SCP) and Deep Capillary Plexuses (DCP) respectively. These changes include reduced foveal and perifoveal vascular density, Non-Perfusion Areas (NPA), capillary oedema and telangiectasia, vascular tortuosity, micro aneurysms of the foveal perivascular plexus disruption and collateral vessel formation [5]. Wide-Field OCT-A (WF-OCT-A) is becoming increasingly popular for detecting neovascularisation in ischemic RVO patients [6]. This technological development of OCT-A will soon assume even greater clinical importance. For example, CRVO in OCT-A is characterized by the presence of clearly visible NPA and areas of oedema with a loose vascular network caused by capillary occlusion and by vascular abnormalities (anastomoses, vascular loops, tortuous and dilated vessels) [7,8].
The limitations of OCT-A are associated with the small scanning area of the retina, segmentation errors resulting from anatomical structural differences of the macula, the inability to detect vascular leaks and artefacts caused by the patient’s movement. The ability to visualize blood flow is also limited, for example, FA-imaged micro aneurysms will not always be visible on OCT-A as the blood flow rate may be below the OCT-A detection threshold [9-11]. Moreover, OCT-A might visualize artefacts related to intraretinal or sub retinal haemorrhages, which appear as of shadows or projection artefacts of superficial vessels in the deeper retinal layers. Nevertheless, these errors are becoming less frequent given the artefact correction modules used in OCT-A equipment [12].
There are few studies on RVO changes on OCT-A after intravitreal administration of the specific anti-VEGF drug aflibercept. In this section, we present our results and compare them with information in the literature.
The NPA observed in RVO are regions without visible capillaries that are more pronounced in the DCP than in the SCP. The NPA result from a lack of blood flow and limitations to its detection when blood flow falls below the detection threshold of OCT-A devices [13,14]. Measuring NPA in the perifoveal area was the most important parameter associated with Best Corrected Visual Acuity (BCVA) in patients with MO. Furthermore, NPA was significantly correlated with peripheral perfusion deficiency [15]. In our study, the mean NFA change in the study's eye was important and totaled 30%. NPA was significantly increased 30 days after the first aflibercept injection and was reduced at subsequent measurement points. This result can be explained by the likely distortion of the result due to increased examination field congestion and retinal swelling and through venous vessel dilatation that partially masked the NPA surface in this disease phase. The NPA was significantly decreased after a therapeutic effect was achieved following aflibercept treatment [16]. A higher number of monthly intravitreal aflibercept injections increased the reduction of the NPA in the superficial and deep retinal layers. Furthermore, blocking the VEGF receptors inhibited NPA deepening by improving vascular flow in the retinal microcirculation and the large areas of non-perfusion strongly correlated with the recurrence of macular oedema (Figures 3a and 3b) [17-19].
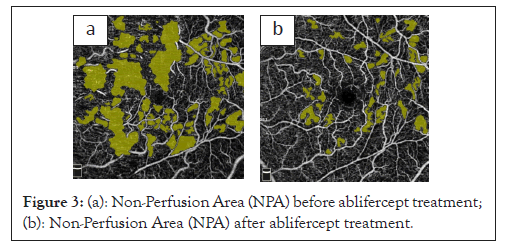
Figure 3: (a): Non-Perfusion Area (NPA) before ablifercept treatment; (b): Non-Perfusion Area (NPA) after ablifercept treatment.
In the study previously published by us, OCT-A assessment of superficial and deep Vascular Density (VD) in the RVO showed that VD was reduced statistically significantly within 30 days of the first medication dose. Observation of the retina after the first aflibercept injection, when swelling had reduced and actual vascularization appeared indicate a positive trend of increasing VD at subsequent measurement points up until the end of the study (Figures 4a and 4b and 5a and 5b).
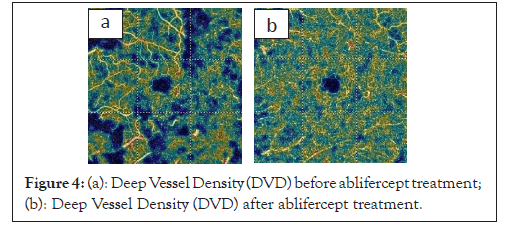
Figure 4: (a): Deep Vessel Density (DVD) before ablifercept treatment; (b): Deep Vessel Density (DVD) after ablifercept treatment.
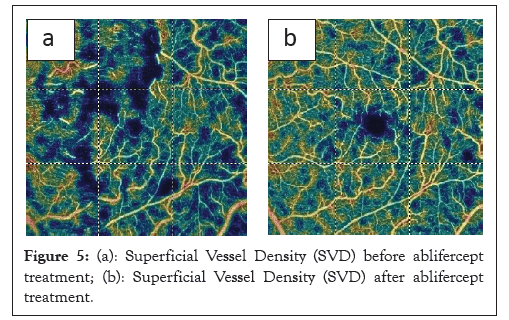
Figure 5: (a): Superficial Vessel Density (SVD) before ablifercept treatment; (b): Superficial Vessel Density (SVD) after ablifercept treatment.
The literature contained discrepancies regarding the effects generated after the anti-VEGF intravitreal injections. The VDSF and VDD might remain unchanged or decrease after treatment. This result is due to the progressive NPA enlargement over time, when the non-ischemic CRVO is converted into the ischemic form [20,21]. Some data indicated increased perifoveal VD after anti-VEGF treatment, while others demonstrated reduced VD in the DCP compared to the SCP [22]. Anti-VEGF treatment significantly reduced the number of interrupted perifoveal capillary arcade networks and qualitatively improved the segmentation of these networks in the SCP and DCP and reduced the number of cystoid spaces in both plexuses [23]. In the DCP, collateral circulation (veno-venous connections) formed as a result of long-term retinal hypoxia [24,25].
An analysis of changes in RVO retinal haemodynamics includes an assessment of the Outer Retinal Flow Area (ORFA) and Choriocapillaris Flow Area (CCFA) variables. In relation to our work, analysis of the CCFA change profiles revealed a statistically significant increase at all measurement points compared to before therapy began. A significant 10% increase in CCFA was observed after the first and second aflibercept intravitreal injections. The ORFA change profiles revealed a statistically significant reduction at all measurement points compared to baseline. The greatest reduction in ORFA (23%) was recorded on day 30 after the first aflibercept injection. Retinal congestion is reduced after the first aflibercept injection and a positive trend of increasing vascular perfusion is revealed at subsequent measurement points, which demonstrates the therapy effectiveness (Figures 6a and 6b and 7a and 7b).
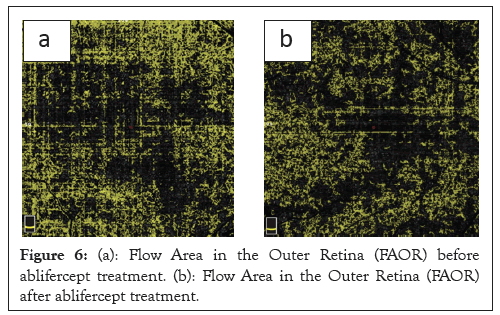
Figure 6: (a): Flow Area in the Outer Retina (FAOR) before ablifercept treatment. (b): Flow Area in the Outer Retina (FAOR) after ablifercept treatment.
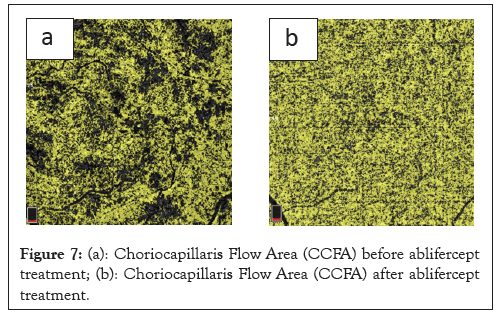
Figure 7: (a): Choriocapillaris Flow Area (CCFA) before ablifercept treatment; (b): Choriocapillaris Flow Area (CCFA) after ablifercept treatment.
Ischemic maculopathy involves the disruption of the perifoveal capillary plexus of the Foveal Avascular Zone (FAZ), more often in the DCP than the SCP. The degree of capillary network rupture correlates with the presence of peripheral ischemia and the degree of non-perfusion in the DCP.
Our earlier results demonstrated that the FAZ range change profile in RVO was characterized by a gradual and statistically significant increase in relation to the baseline values. The FAZ change was highest between day 30 after the third aflibercept injection and the pre-treatment value. The lowest FAZ change dynamic occurred between day 90 and 30 after the third aflibercept injection. Therefore, the therapeutic effect as measured using the FAZ value could be observed during the aflibercept treatment and remained at a similar level until the end of the study (Figures 8a and 8b).
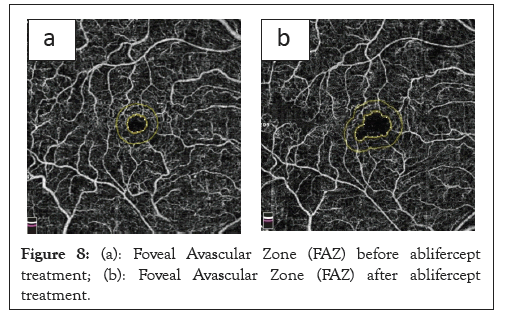
Figure 8: (a): Foveal Avascular Zone (FAZ) before ablifercept treatment; (b): Foveal Avascular Zone (FAZ) after ablifercept treatment.
Many researchers believe that the FAZ should be assessed only once MO has subsided, which artificially enlarges it as intra retinal cysts displace the central vascular network, masking the actual FAZ surface. FAZ re-modelling might suggest dynamic changes to the RVO vascular flow. When compared with the smaller, more limited area of the FAZ, the higher number of anti- VEGF injections reduce and slows its progression [26-29].
The FAZ size and macular VD correlate with the size of the peripheral NPA. The boundary between the FAZ and the adjacent NPA is obscured in RVO, which might indicate deterioration in the OCT-A imaged clinical condition. Reduced retinal VD might indicate the development of retinal neovascularisation. CRVO features a larger FAZ than BRVO and its size might be related to the intraocular VEGF level.
The dilation of the venous vessels and the capillary network, mainly DCP, is probably caused by increased endovascular resistance and increased concentrations of VEGF vascular growth factor and pro-inflammatory cytokines due to retinal ischemia and hypoxia [30].
OCT-A can also observe and assess anatomical abnormalities during RVO, such as micro aneurysms, cystoid MO, intra retinal haemorrhages, vascular malformations and neovascularisation. Micro aneurysms occur more frequently in BRVO and form on the border of NPA and dilated venous vessels, especially within the DCP. Micro aneurysms cause persistent vascular leakage and MO after the elevated venous pressure has subsided [31]. Cystic oedema is more frequently localized in the SCP in CRVO and in the DCP in BRVO. Intra-retinal cysts overlap with NPA and the resulting capillary dislocation improves perfusion indicators after treatment. Where perfused hyper-reflective foci are absent, cysts are characterized by high lipid concentrations, which are predictors of vision deterioration. Intra-retinal haemorrhages produce an optical shadow on OCT and their location might obscure the superficial or deep vascular plexus. Ghost vessels are visible in en face OCT but are not detectable in OCT-A or FA [32]. Optic nerve disc malformations and neovascularization (new vessels on the disc, NVD) form at the superficial peripapillary plexus level and are visible as vascular loops. Neovascularization’s are located above the retina at the vitreous body level and are visible as a network of small blood vessels [33]. Neovascularisation outside the optic disc (neovascularisation elsewhere NVE) is clearly visible on OCT-A, which enables the monitoring of new vessel formation and treatment assessment.
Based on published research, it can be concluded that the parameters measured in OCT-A and BCVA are correlated and our results were consistent with those in the literature. BCVA is the clinical result of the haemodynamic variables presented above. We observed significantly higher BCVA values in the study group throughout the entire observation period compared to the pre-treatment value. Aflibercept treatment improved the BCVA of RVO patients. The factors related to BCVA are FAZ size, FAZ area in the superficial and deep vascular plexuses, NPA and vascular perfusion in the DCP [34-37]. The extent of the FAZ area and dimensions in the deep retinal plexus are inversely related to visual acuity, enlargement of the FAZ area was associated with reduced visual acuity [38,39]. Intravitreal anti-VEGF treatment significantly improved the final averaged BCVA value, with successive gradual improvements during further treatment. Additionally, SCP and DCP vascular perfusion in RVO correlated positively with the final BCVA. Long-term clinical observations of RVO patients reported relative stabilization of visual acuity after subsequent anti-VEGF injections, which coincided with improved hemodynamic parameters assessed using OCT-A. In RVO patients, microcirculation changes lead to MO and worsen visual acuity. Improved visual acuity after the treatment of MO correlated strongly with improved retinal perfusion and reduced degrees of ischemia. Persistent retinal ischemia might irreversibly damage its structure and function, resulting in the irreversible deterioration of visual acuity. In turn, the reduced retinal VD might indicate the development of retinal neovascularisation.
Intra-vitreal injections of anti-VEGF drugs, including aflibercept, have revolutionized the treatment of many retinal diseases, including macular oedema associated with RVO. Aflibercept therapy improves retinal anatomy and provides functional benefits for retinal microcirculation. It is particularly important in anti-VEGF drug therapy to start treatment as early as possible.
OCT-A is the latest non-invasive technique for imaging retinal and choroidal vessel structures and their blood flow. OCT-A is used for many conditions, including RVO. OCT-A enables a detailed analysis of the central macular area and evaluation of the following parameters VDSF and VDD, ORFA, CCFA, NPA and the FAZ. OCT-A monitoring revealed that treating RVO with intra-vitreal injections of an anti-VEGF drug reduced MO and NPA, increased VD and improved retinal and choroidal blood flow and visual acuity. OCTA will not only help to understand pathology and changes in the complex microvasculature of macula after RVO, but in the future, it may play a major role in management of RVO patients.
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar ] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar ] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar ] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar ] [PubMed]
Citation: Spiewak D, Drzyzga L, Dorecka M, Wygledowska-Promienska D (2024) Evaluation of Microcirculation Parameters in Optical Coherence Tomography Angiography in Retinal Vein Occlusion Before and After Aflibercept Treatment: A Review. J Clin Exp Ophthalmol. 15:973.
Received: 26-Feb-2024, Manuscript No. JCEO-24-29782; Editor assigned: 28-Feb-2024, Pre QC No. JCEO-24-29782 (PQ); Reviewed: 13-Mar-2024, QC No. JCEO-24-29782; Revised: 20-Mar-2024, Manuscript No. JCEO-24-29782 (R); Published: 27-Mar-2024 , DOI: 10.35248/2155-9570.24.15.973
Copyright: © 2024 Spiewak D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.