Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2010) Volume 1, Issue 1
In the present study, the crude ethanol extracts of Calotropis procera and Annona squamosa leaves have been screened for their larvicidal activities against Musca domestica. The third instar larvae of housefly were treated with the different concentrations of both the extracts by dipping method for 48 h. The LC50 values of the extracts of C. procera and A. squamosa leaves were found to be 282.5 and 550 mgl-1, respectively. The phytochemical analysis of these extracts suggested presence of alkaloids as the major component. The larvae were exposed to 5 and 10% concentrations of the LC50 value of each extract along with their control sets to evaluate their effect on metamorphosis, nucleic acid and protein content in different developmental stages. The leaf extract of C. procera was found to be more active in terms of insecticidal potential. The data indicate that the leaf extracts of these plants may be utilized as the probable candidates for the development of bioinsecticides to control the population of Musca domestica as safer and economic alternatives to the synthetic insecticides.
<Keywords: Musca domestica, Calotropis procera, Annona squamosa, Bioinsecticide, LC50 .
With a greater awareness of the hazards associated with the use of synthetic organic insecticides, there has been an urgent need to explore suitable alternative products for pest control. Screening of plant extracts for deleterious effect on insects is one of the approaches in the search of novel biological insecticides [1]. Insecticidal activity of many plants against several insects has been demonstrated [2,3]. Seed as well as foliar extracts of several plants have been reported to have toxic and potent growth reducing activity to insects [4]. The deleterious effect of plant extracts or pure natural/ synthetic compounds on insects can be manifested in several manners including toxicity, mortality, antifeedant, growth inhibitor, suppression of reproductive behavior and reduction of fecundity and fertility.
Exposure of toxic agents to animals can cause changes even at the molecular level. Nucleic acids (DNA and RNA) and protein contents are regarded as important biomarkers of the metabolic potential of cells, as they play the main role in regulating the different activities of cells. Changes in the amount of nucleic acid can be used to detect whether toxic agents affect cellular proliferation and cell death [5,6,7].
The common housefly Musca domestica (Diptera:Muscidae) is an important mechanical vector of several bacterial and pathogenic organisms of human and animals [8]. Recently some reports have indicated that on prolonged exposure to chemical insecticides worldwide, houseflies have developed resistance to them e.g. spinasod [9], diflubenzuron [10] and synthetic insecticides [11].
Calotropis procera is a member of the plant family, Asclepiadaceae, a shrub widely distributed in West Africa, Asia and other parts of the tropics. The plant is erect, tall, large, branched and perennial with milky latex throughout .A large quantity of latex can be easily collected from its green parts [12]. Local people use it successfully to combat some cutaneous fungal infections. The abundance of latex (containing alkaloids) in the green parts of the plant reinforces the idea that it produced and accumulated latex as a defence strategy against organisms such as virus, fungi and insects [13]. The presence of plant defence related proteins such as hevein, an alpha-amylase inhibitor has been described to occur in the latex secretion of other plants [14]. However there are no reports to indicate that Calotropis leaves and seed extracts exhibit insecticidal properties against Musca domestica.
The Annonaceae (Custard- apple family) is a large family of almost exclusively tropical trees and shrubs comprising about 130 genera and 2300 species. Some plants of this family have been used traditionally as insecticides [15]. For example, the powdered seeds and leaf juices of Annona spp. are used to kill head and body lice, and bark extract of Goniothalamus macrophyllus is used as mosquito repellents. Annonaceous acetogenins extracted from the tree leaves, bark and seeds have pesticidal and/or insect antifeedant properties [16]. However there is no report to indicate that Annona squamosa extract possess insecticidal activity against housefly.
Keeping these facts in view, the present study was undertaken to investigate the larvicidal activities of ethanolic extracts of C. procera and A. squamosa leaves under laboratory conditions as well to assess the chemical nature of the active components present in the extracts along with their effect on nucleic acids and protein content in different developmental stages of housefly.
Rearing technique
Adult house flies were collected from local areas using a sweep net and reared in the laboratory at 26±2°C, 60±10% RH, photoperiod 12:12 (L:D). The rearing method described by Kristensen and Jespersen [10] was adopted in the present study. In brief, the adults of M. domestica were fed milk and dry sugar. Mixture of wheat flour and milk was prepared at a weight ratio of 1:3 and 35g of this mixture was placed on a Petri dish with wet cotton as an oviposition site.
Collection and processing of plants sample
Fresh leaves of C. procera and A. squamosa were collected from the Botanical Garden of University of Allahabad. Leaves were properly cleaned and shade dried for 5-7 days at 32-35°C and relative humidity 50- 60%. The dried leaves were powdered mechanically using commercial electrical stainless steel blender (Remi Anupam Mixie Ltd., India). The samples were stored in air tight container at room temperature in dark for further analysis.
Extraction of plant extracts
The dried leaves of C. procera and A. squamosa were extracted with 1 litre of 90% ethanol in a Soxhlet apparatus (Borosil, India) using the method described by Mishra et al.[17] The extracts were concentrated at 50°C and the residue obtained was stored at 4°C.
Phytochemical analysis of the extract
Qualitative phytochemical analysis of leaf extracts of C. procera and A. squamosa was done by the method of Mishra et al. [17]. In brief, the phytochemicals such as tannins, alkaloids, saponins, flavonoids, terpenoids and phenols/polyphenols were qualitatively determined as following:
Tannins
Twenty mg extract was dissolved in 2 ml distilled water and filtered. Two ml FeCl3 was added to the filtrate, blue-black precipitate indicated the presence of tannins.
Alkaloids
20 mg extract was dissolved in 2 ml distilled water and filtered. To the filtrate, 2–4 drops of 1% HCl was added and steam was passed through it. To the 1 ml of this solution 6 drops of Wagner’s reagent was added. Brownish-red precipitate indicated the presence of alkaloids.
Saponins
To 0.5 ml of the filtrate obtained in alkaloids test 5 ml distilled water was added. Frothing persistence indicated the presence of saponins.
Flavonoids
20 mg extract was dissolved in 10 ml ethanol and filtered 0.5 ml conc. HCl and magnesium ribbon were added to 2ml filtrate. Development of pink-tomato red color indicated the presence of flavonoids.
Terpenoids
Salkovski test was performed using a small amount of extract solution. To this solution 5 drops of conc. H2SO4 and 1 ml Chloroform were added. Change of yellow colour into red indicated the presence of terpenoids.
Phenols/polyphenols
A small amount of material was extracted in ethanol and evaporated to dryness. Residue was dissolved in distilled water and 0.5 ml Folinciocalteau reagent was added followed by 2 ml 20% Na2CO3 solution. Development of bluish color indicated the presence of phenols.
Preparation of experimental concentrations: Stock solution was prepared by dissolving 5 mg extract in 10 ml of ethanol as the Calotropis extract does not dissolve in water. This solution was used for making further dilutions. The different extract concentrations such as 100, 200, 300, 400 and 500 ppm were used for further study following trial runs with various concentrations of the extract. In case of A. squamosa, the extract concentrations were 200, 400, 600, 800 and 1000 ppm.
Method of treatment
Twenty number of late 3rd instar larvae of M. domestica identified by shortening and thickening of size and shape, respectively, darker in color and presence of three spiracles at the posterior end of the body [18] were selected for each set of treatment. Seven numbers of glass beakers of 250 ml capacity were taken and labeled for different concentrations in addition to one for check and one for control. In case of control, water and for check, ethanol was added in place of extract. Larvae were dipped into the solution for two minutes and then transferred back in the rearing medium (composition mentioned above). Each experiment was conducted in triplicates along with the control group. Mortality of larvae followed by the exposure was recorded after 24h up to 48h. LC50 was calculated using Karber’s method [19]. In brief, the mortality due to treatment of 3rd instar larvae with different concentrations of the leaf extracts of the two plants was recorded after 24 and 48 h. The LC50 value was determined as following:
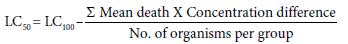
The experiment was repeated three times on subsequent days. Same method of treatment was applied for both the extracts. Morphological appearance of the resultant pupae and the subsequent emergence of the adults from it were also observed. To evaluate the insecticidal effect of the leaf extracts of C. procera and A. squamosa, the 3rd instar larvae of M. domestica were treated with 5% and 10% (equivalent to 1/20 and 1/10 of LC50 value) for 24 and 48h. The control group was exposed only with the equal volume of ethanol, the solvent in which the extract was prepared.
Homogenate preparation
After 48 h of exposure larvae were homogenized (10%, w/v) in 50 mM Tris–HCl buffer (pH 7.5) on ice using Potter-Elvehjam homogenizer fitted with a Teflon-coated pestle (Remi, India). The homogenates were centrifuged at 4°C for 10 min at 15,000 g in a refrigerated centrifuge (Sigma, model-3K30, Germany). The corresponding supernatants were either used fresh or kept frozen at -20°C until further use for determining the concentration of different biomolecules.
Total protein content estimation
Protein levels were estimated following the method of Lowry et al. [20] using bovine serum albumin as standard. In brief, the protein samples (tissue extracts) were mixed with the protein reagent (alkaline copper sulphate solution), incubated for 10 min at room temperature followed by addition of Folin-ciocalteau reagent. The reaction mixture was further incubated at room temperature for 30 min. The absorbance of blue color was monitored at 660 nm using spectrophotometer (Systronics Visiscan 167, India). Simultaneously, a blank was also processed containing all the reagents except the protein. Bovine serum albumin (BSA) was used as a standard.
Extraction and estimation of nucleic acids
Nucleic acids (DNA and RNA) were extracted using the method described by Schneider [21]. Calf thymus DNA was used as a standard. Total RNA was estimated by the orcinol method [21] using yeast RNA as a standard.
Statistical analysis
All values in the table are given as mean ± standard error of mean (SEM) of three independent experiments. Graph pad software was used to analyze the data.
The results presented in (Tables 1 and 3) exhibit the toxicity of Calotropis and Annona leaf extracts against M. domestica larvae, respectively. The treatment of 3rd instar larvae of M. domestica with different concentrations of the leaf extracts of these two plants exhibited relatively lower percent mortality after shorter duration (24h) than that at longer duration (48h). The exposure of the flies to both of the ethanol extracts caused significant mortality in a dose dependent manner. A. squamosa leaf extract exhibited similar level of toxicity to the 3rd instar larvae of M. domestica at 200 ppm concentration of the leaf extract with that of C. procera causing 20% mortality after 48h. The ethanol extract of C. procera was found to be quite effective against M. domestica larvae as nearly 100% mortality was observed at 500ppm. The mortality curves for the determination of LC50 values for the ethanol extracts of C. procera and A. squamosa are shown in Figures 1 and 3, respectively. The LC50 values of C. procera and A. squamosa extracts were calculated to be 282.5 and 550 ppm, respectively (Tables 2 and 4). Both the extracts showed antifeedant activity leading to weight loss and reduced size of the treated larvae as compared to control ones.
| Concentration (ppm) |
No. of live Larvae |
% Alive at 24 h 48 h |
%Mortality at 24 h 48 h |
|
|
24 h |
48 h |
|||
|
0 (control) 0 (check) 100 200 300 400 500 |
20 20 19 17 12 10 05 |
20 20 17 14 9 8 0 |
100 100 100 100 95 85 85 80 60 45 50 40 25 0 |
0 0 0 0 5 15 15 20 40 55 50 60 75 100 |
The larvae of M. domestica (20 in each set) were treated with different concentrations of ethanolic extract of leaves of C. procera for 24 and 48 h as shown in Materials and Methods. The control and check represent larval treatment with water and ethanol, respectively. The experiments were conducted in triplicate
Table 1: Toxicity testing of the ethanol extract of Calotropis procera leaves.
|
Concentration |
Concentration difference |
No. of alive larvae |
No. of dead larvae |
Mean |
Mean death x |
|
00 (control) 00 (check) 100 200 300 400 500 |
0 0 100 100 100 100 100 |
20 20 17 14 9 8 0 |
0 0 3 6 11 12 20 |
0 0 3.0 4.5 8.5 11.5 16.0 |
0 0 300 450 850 1150 1600 |
|
4350 |
|||||
The LC50 value of the leaf extract of Calotropis procera for 48 h has been determined according to the arithmetic method of Karber (1931). The calculation was done as following:
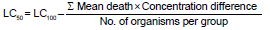
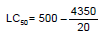
LC50 = 500 – 217.5
LC50 = 282.5ppm
Table 2: LC50 value of the leaf extract of Calotropis procera for 48 h.
|
Concentration (ppm) |
No. of live Larvae |
% Alive at |
% Mortality at |
|
| 24 h | 48 h | |||
|
0 (control) 0 (check) 200 400 600 800 1000 |
20 20 19 17 15 13 8 |
20 20 16 13 10 6 0 |
100 100 100 100 95 80 85 65 75 50 65 30 40 0 |
0 0 0 0 5 20 15 35 25 50 35 70 60 100 |
The larvae of M. domestica (20 in each set) were treated with different concentrations of ethanolic extract of A.squamosa leaves for 24 and 48 h as shown in Materials and Methods. The control and check represent larval treatment with water and ethanol, respectively. The experiments were conducted in triplicate
Table 3: Toxicity testing of ethanol extract of Annona squamosa leaves against M. domestica.
|
Concentration (ppm) |
Concentration difference |
No. of alive larvae |
No. of dead larvae |
Mean |
Mean death x |
|
0 (control) 0 (check) 200 400 600 800 1000 |
0 0 200 200 200 200 200
|
20 20 16 13 10 06 0
|
0 0 4 7 10 14 20
|
0 0 2 5.5 8.5 12 17
|
0 0 400 1100 1700 2400 3400 |
|
Total 9,000 |
|||||
The LC50 value of the leaf extract of Annona squamosa for 48 h has been determined according to the arithmetic method of Karber (1931). The calculation was done as following:
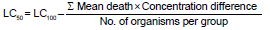
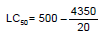
LC50 = 1000 – 450
LC50 = 550 ppm
Table 4: The LC50 value of ethanol extract of Annona squamosa leaves for 48 h.
Figure 2: Musca domestica pupae showing the morphological changes due to the treatment with the leaf extract of Calotropis procera under experimental conditions as mentioned in the Materials and Methods. a –Treated with water (Control) or ethanol (check) for 2 min daily for two days b –Treated with C. procera leaf extract daily for two days.
Pupal deformities followed by C. procera treatment included dorsoventral compression, reduction in size, collapsed appendages and their failure to metamorphose into adults. Pupae with certain larval characters were also observed (Figure.2).
The morphological changes like reduced size, darkening of the puparium, condensed appendages and failure to metamorphose were recorded due to the treatment of A. squamosa leaf extract. Other morphological appearances of the pupae were not much different from that of control excepting that of reduction in size (Figure.4). The results clearly indicated that the mortality of the larvae in both the cases was dose dependent. It was observed that during the course of metamorphosis, the tendency for the development of test larvae to pupae decreased with a rise in the concentration of the ethanol extracts (Table 5). The larvae treated with the extract of C. procera leaves at 500 mg/l exhibited 100% mortality. At lower concentration, some larvae could continuously develop. Similarly, no pupa formation was recorded in case of 1000 mg/l concentration of ethanol extract of A. squamosa as it caused 100% mortality of the larvae during the exposure period. In the untreated condition, most of these larvae developed to pupae within 5 days but the pupation period got delayed up to 6-7 days due to treatment with these extracts.
Figure 4: Musca domestica pupae showing the morphological changes due to the treatment with Annona squamosa leaf extract under experimental conditions as mentioned in the Materials and Methods. a –Treated with water (Control) or ethanol (check) for 2 min daily for two days b –Treated with Annona squamosa leaf extract daily for two days.
The rate of emergence of adults was also significantly inhibited due to the treatment of both of these extracts and the impact was concentration dependent. The results suggest inhibition of adult emergence by 12.50% due to the treatment of C. procera extract (400 mg/l), whereas 33.33% emergence was recorded at 800mg/l concentration of A. squamosa extract. However, at highest concentrations (500 and 1000 mg/l, respectively) of C. procera and A. squamosa extracts, no emergence was observed (Table 5). In the control set of experiments, the adults emerged from pupae within eight days but in case of treated groups the pupation period got delayed resulting in emergence of the adults on 10-11th day.
|
Ethanol extract from plant leaves |
Concentration |
Percentage of larvae |
Percentage of pupae emerged into adult ** |
|
Calotropis procera |
0 |
100% (20/20) |
100% (20/20) |
|
Annona squamosa |
0 |
100%(20/20) |
100% (20/20) |
*Each value in parenthesis is the number of total pupae developed from the larvae/ total number of larvae
**Each value in parenthesis is the number of pupae developed to adults/the number of total pupae
Table 5: Effect of Calotropis procera and Annona squamosa leaf ethanol extracts on the metamorphosis of Musca domestica.
The phytochemical analysis of the extract (Table 6) showed the presence of alkaloids in maximum amount in ethanol extract of C. procera leaves, which was higher than that in A. squamosa. The phenolic contents were also found but in lower quantity in both the extracts. In addition, the flavonoids and terpenoids were also found to be present in ethanol extract of A. squamosa in traces. These two compounds were found to be missing from C. procera extract as determined by the method used in this study. Tannins and Saponins could not be detected in both of these extracts.
| Chemicals | Calotropis procera | Annona squamosa |
| Tannins | _ | _ |
| Flavonoids | _ | + |
| Tarpenoids | _ | + |
| Phenols | + + | + |
| Alkaloids | + + + | + + |
| Saponins | _ | _ |
The phytochemicals in the ethanol extracts of the Calotropis procera and Annona squamosa leaves were determined as described in Materials and Methods. - absent; + moderately present; ++ highly present; +++ very highly present
Table 6: Phytochemical analysis of ethanol extracts of Calotropis procera and Annona squamosa leaves.
DNA, RNA and protein levels were reduced significantly (p<0.5) by both plant extracts in the high concentration after 48 h of exposure for stages (larva, pupa, adult) of M. domestica (Tables 7, 8).
| Stage | DNA mg/g wet wt. of tissue | RNA mg/g wet wt. of tissue | Protein mg/g wet wt. of tissue | ||||||
| C | 5% | 10% | C | 5% | 10% | C | 5% | 10% | |
| Larva | 0.382 ±0.0007 |
1.911** ±0.00 (-49.98) |
0.564* ±0.0015 (-85.71) |
0.241 ±0.002 |
0.225** ±0.0030 (-6.64) |
0.182** ±0.0019 (-24.49) |
415.22 ± 0.00 |
291.51** ±0.002 (-29.8) |
290.35** ± 0.002 (-30.1) |
| Pupa | 1.446 ±0.002 |
0.873* ±0.0015 (-39.63) |
0.573** ±0.0035 (-60.38) |
0.168 ±0.00 |
0.163ns ±0.0045 (-2.98) |
0.108** ±0.0115 (-35.72) |
309.63 ±0.0064 |
261.37** ±0.0075 (-15.59) |
240.50** ±0.0035 (-22.31) |
| Adult | 1.719 ±0.0015 |
1.416ns ± 0.002 (-17.63) |
1.392** ±0.00 (-19.03) |
0.159 ±0.0015 |
0.102* ±0.0075 (-35.85) |
0.089** ±0.0455 (-44.03) |
299.12 ±0.0065 |
147.75** ±0.0075 (-50.61) |
136.75** ±0.0035 (-54.29) |
Data were analyzed by Graphpad software,, values given as mean ± SEM Values in parentheses are percent change over control. Significance (*) of data is shown in superscripts, ns P>0.05(non significant), *P<0.05(significant), **P<0.01(very significant), C-Control (0%), 5% & 10% are 1/20 and 1/10 of LC50
Table 7: Effect of ethanol extract of Calotropis. procera leaf on the level of nucleic acids and protein after 48 h exposure of Musca domestica.
| Stage | DNA mg/g wet wt. of tissue | RNA mg/g wet wt. of tissue | Protein mg/g wet wt. of tissue | ||||||
| C | 5% | 10% | C | 5% | 10% | C | 5% | 10% | |
| Larva | 0.382 ±0.0007 |
0.213* ±0.0017 (-35.06) |
0.134* ±0.002 (-59.15) |
0.241 ±0.002 |
0.237* ±0.0017 (-1.65) |
0.211** ±0.0023 (-12.45) |
415.22 ± 0.00 |
332.70* ±0.002 (-19.92) |
311.56** ± 0.0019 (-24.97) |
| Pupa | 1.446 ± 0.002 |
0.935* ±0.0017 (-39.63) |
0.611** ±0.003 (-60.38) |
0.168 ±0.00 |
0.165ns ±0.0024 (-1.79) |
0.135* ±0.003 (-19.64) |
309.63 ±0.0064 |
280.32 ns ±0.0052 (-9.47) |
263.54* ±0.0039 (-14.89) |
| Adult | 1.719 ±0.0015 |
1.493ns ± 0.002 (-17.63) |
1.429* ±0.001 (-16.87) |
0.159 ±0.0015 |
0.135 ns ±0.0041 (-15.09) |
0.117* ±0.0027 (-26.42) |
299.12 ±0.0065 |
173.79** ±0.0059 (-42.03) |
166.05** ±0.0048 (-49.49) |
Data were analyzed by Graphpad software,, values given as mean ± SEM Values in parentheses are percent change over control. Significance(*) of data is shown in superscripts, ns P>0.05(non significant), *P<0.05(significant), **P<0.01(very significant), C-Control (0%), 5% & 10% are 1/20 and 1/10 of LC50
Table 8: Effect of ethanol extract of Annona squamosa leaf on the level of nucleic acids and protein after 48 h exposure of Musca domestica.
In the present study, the ethanol extracts of the leaves of the plants, C. procera and A. squamosa, were quite effective against the housefly larvae. These extracts drastically affected the pupation and emergence of the adults from pupae in dose dependent manner.
Previous investigations on annonaceous acetogenin, the bioactive principle of the plant family Annonaceae, have shown that it may have pesticidal or antifeedant properties [22, 23]. Seed oil of A. squamosa has been reported to reduce survival of leaf hopper, Nephotettix virescens (Hemiptera: Cicadellidae) and transmission of rice tungro virus [24, 25].
Though reports on nematicidal [26], antimicrobial and antihelminthic [27] activities of C. procera extract and its use in the treatment of toothache, cough and subcutaneous diseases [28] exist, there is no report at all regarding the LC50 for the alcoholic extract of C. procera leaf against M. domestica. The laboratory study on larvicidal properties of leaf extract of C. procera against mosquito larvae is known [29]. The results from the present study indicate the antifeedant property of the Calotropis extract which may be due to the different compounds present in the extract possessing different bioactivities.
As shown in (Table 5), the alterations in the rate of pupation and adult emergence due to treatment of the plant extracts were dose dependent. This may be due to the effect of some active ingredients present in the extracts which exhibit potential to cause interference into the normal metabolism of the insects [30].
The phytochemical analysis (Table 6) of the two extracts revealed the presence of alkaloids and phenols only in C. procera leaf extract whereas in A. squamosa, the presence of alkaloids, phenols, flavonoids and tarpenoids was detected. These phytochemicals may be responsible for the insecticidal nature of the extracts. Previously from the leaves of A. squamosa, several flavonoids [31] and a tetrahydroisoquinoline alkaloid with cardiotonic activity [32] have been isolated. Partially purified flavonoids of aqueous A. squamosa leaves extract have been demonstrated to possess antimicrobial and insecticidal activity [33]. Similarly, the alkaloids reported to be present in the latex of C. procera have been shown to contain insecticidal properties [13].
Similarly, when 6th instar larvae of Tribolium castaneum were treated with higher doses of fenpropathrin, RNA and DNA contents were reduced by 21% and 20%, respectively [34].
Nucleic acids and protein contents are regarded as important biomarkers of the metabolic potential of cells, as these play the main role in regulating the different activities of cells. Since insects have very little carbohydrate, protein is used to meet the increased energy demand. Proteins are mainly involved in the architecture of the cell which is the chief source of nitrogenous metabolism.
The decreases in total protein level in the different developing stages suggest the high protein hydrolytic activity due to elevation of protease activity. Inhibition of DNA synthesis, thus, might affect both protein as well as protein synthesis machinery. The results of this study suggest that the extracted compound (s) is a potent inhibitor of DNA synthesis, which in turn results in the reduction of RNA level. Carbohydrates are the primary and immediate sources of energy. In stress condition, carbohydrate reserve is depleted to meet energy demand.
The data obtained from the present study clearly indicate that C. procera and A. squamosa leaf extracts were quite effective as larvicides for providing a better and excellent alternate for the control of M. domestica. However, isolation of the active compounds from these plants and further trial assay in the field conditions is underway to explore its suitability for such application.
The authors express their gratitude to Prof. P. Gaur, Ex-Head, Department of Zoology, University of Allahabad for providing necessary facilities. Financial assistance to one of the authors (NB) from the University of Allahabad (UGC) and UGC-SAP (RFSMS) to the Department of Zoology is gratefully acknowledged.