Journal of Plant Biochemistry & Physiology
Open Access
ISSN: 2329-9029
ISSN: 2329-9029
Research Article - (2022)Volume 10, Issue 10
Microspore embryogenesis is the developmental plasticity of juvenile male gametophytes to switch from pollen to embryo development upon exposure to in vitro stress. It is a common method for obtaining haploid and doubled haploid plants in breeding programs for development of superior varieties via haploid-diploidization which allows fast development of homozygous lines from heterozygous parents. In cassava, obtaining haploidization through traditional methods of successive selfing is difficult because of cassava’s long reproductive cycle, high heterozygosity, and inbreeding depression. As a first step towards microspore embryogenesis, callus induction following heat treatment has not been investigated in cassava. We used two elite Ugandan cassava varieties, hereafter called “genotypes”, NASE3 and NASE14, for the study of callus induction. Heat stress of 40°C for 0, 6, 12, 18 and 24hrs and Murashige and Skoog medium supplemented with 2-9% sucrose, and 2,4-dichlorophenoxy acetic acid were used. Heating anthers of NASE3 at 40°C for 6 hrs resulted in a significantly higher percentage of callus induction on MS medium supplemented with 2% sucrose. Callus emerged from inside of the anthers with production influenced by genotype, sucrose concentration, anther density and duration in culture (P≤0.001). Limited in vitro callus differentiation was observed on auxin-and cytokinin-supplemented media. In both genotypes, embryo genic callus was obtained in liquid medium, while green callus was achieved on solid medium. This is a significant step upstream of double haploid plant production pathway in cassava: rigorous optimization of protocols downstream of callus induction are needed for regeneration of microspore-derived embryos and haploid plants.
Cassava (Manihot esculenta Crantz) can grow and perform on marginal soils with minimum farm inputs and still yield up to 40-50 tons ha-1 year-1 [1]. In addition, high tolerance to climatic changes [2] and suitability for pieace-meal harvesting make cassava a crop of choice in the fight against hunger in sub-Sahara Africa. Moreover, cassava is an important source of starch contained in the storage roots for many downstream industrial applications [3]. The increasing demand of cassava roots, therefore, requires faster means of generating new genotypes amenable to food/feed or industrial needs. Cassava breeding holds a great promise in providing improved genotypes suitable for various uses and environments [4].
Naturally, cassava is an out-crossing monoecious species, a sexual reproduction strategy that is associated with high heterozygosity. High heterozygosity in cassava is a constraint to the genetic improvement of cassava through conventional breeding methods because many rounds of backcrossing must be undertaken prior to overcome unwanted traits [5]. Under such circumstances, production of homozygous cassava lines would enhance genetic improvement and allow faster production of new genotypes for diverse needs [4]. One of the means through which homozygous cassava lines can be produced, is the use of isolated microspore culture (IMC). IMC is a unique system in plants during cell development, where a particular stress treatment results in reprogramming of microspores by switching from the default developmental gametophytic pathway into embryogenesis [6]. In this process, named androgenesis, plant regeneration is archived from microspore culture under in vitro conditions in which the normal development into pollen cells is stopped and instead force their development directly into a complete plant [7,8]. This system can promote the production of homozygous lines in cassava and significantly accelerate the production of cassava hybrids because in a single generation, doubled haploid (DH) techniques can be used to produce homozygous lines. Apart from use in production of hybrids, homozygous lines have numerous applications such as the ease to generate induced mutations to study gene function, chromosome reduction of polyploid species, gene or quantitative trait loci mapping, and other genomics studies [9].
Usually, development of inbred-lines requires several cycles of inbreeding. However, using anther culture, the process can be reduced to a single generation, which can be equivalent to a year of cassava growth under field conditions. The first bottleneck is in the ability to regenerate haploid plants from microspores in the anthers which determines the effectiveness of the anther culture technique to be employed. The second bottleneck is the capacity to convert the haploids to doubled haploids.
Microspore culture results in true-breeding lines in the subsequent generation. Like other haploid-inducing techniques, androgenesis is genotype-dependent, and can be influenced by the physiological state and growth conditions of donor plants. The growth stage of male gametes, pretreatment of flower buds or anthers also determine the success of this method. The composition of the culture medium, and physical factors during culture together with their interactions also determines the success of the method [10]. Physiochemical factors cause stress responses that halt microspore development or young pollen grains in their gametophytic pathway. Such factors include temperature pre-treatment, sucrose and nitrogen starvation, and osmotic stress [11].
In cassava, the limited reports on anther culture show that it is possible to produce callus [12] and the difficulty in generating haploid plants. However, whereas no plant regeneration was achieved, the induction of cassava microspores towards sporophytic development is possible [13]. The aim of this study, therefore, was to develop a reproducible protocol to induce callus from the microspores enclosed in anther lobes as a first step towards development of doubled haploid plants in cassava via anther culture.
Cassava plants and growth conditions
Two elite ‘Ugandan’ cassava genotypes, NASE3 and NASE14 (used as the donor plants for the anthers) were grown at National Crops Resources Research Institute (NaCRRI), Namulonge, Uganda. These genotypes were selected because of their ability to flower profusely an also being preferred by farmers. To have flowers all year round, plants were established in a staggered fashion at 15-day intervals. Fields were generally managed according to local cassava production practices with weeding done using a hand-held hoe. No application of agrochemicals was done [14].
Flower bud sterilization
To keep fresh, inflorescences harboring male flower buds were collected from the field early in the morning before sunrise (06:00-07:30 hours) and transferred to the laboratory. In the laboratory, male flower buds of 2.0-2.8 mm in diameter containing developmental stages of tetrads were isolated according to Perera et al. (2013). The isolated flower buds were subsequently washed under running tap water and soap and then dried on filter papers for 10 min. To surface sterilize the buds, dried flower buds were placed in a laminar flow hood and placed in 70% ethanol for 1 min, followed by 10% (v/v) sodium hypochlorite (NaOCl) solution (Jik) supplemented with 60 μl/L Tween 20 for 20 minutes. Using sterile water, the disinfected flower buds were then washed four times for 2 minutes each.
Heat stress treatment and anther culture
To subject the male flower buds to heat shock treatment, the flower buds were sealed in the Petri dishes using parafilm and wrapped in aluminum foil while ensuring air-, light-, and watertight conditions inside the sealed Petri dishes. A water bath was set up at 40˚C into which sealed Petri dishes containing the male flower buds were submerged for 6, 12, 18 and 24 hours. Metallic weights of 200g were placed on top of the Petri dishes to keep them submerged in under hot water. Untreated buds (0 hours) were used as controls.
Thereafter, buds were dissected to release the anthers of different densities of 20, 40, 60, 80 and 100 anthers per plate (equivalent to 2, 4, 6, 8, 10 male flower buds in each case). The anthers were cultured on a modified Murashige and Skoog (MS) medium [15] similar to that used by Lentini et al. 1995 [16]. MS medium was supplemented with 2-5mg/L 2,4-dichlorophenoxyacetic acid (2,4- D), 800 mg/L glutamine and varied concentrations of sucrose 2-9% (w/v) with the pH adjusted to 5.8 before autoclaving for 15 min, at 120 °C. After autoclaving, 100 mg/L 2-(N-morpholino) ethanesulfonic acid was added to the culture medium and used for culturing the anthers. The rates of callus formation were calculated by counting the number of anthers that became enlarged, formed callus and later embryos relative to the starting number of cultured anthers.
Five replicates were considered per treatment for each experiment. Anthers were cultured on a 60 × 15cm plate, using MS medium supplemented with 2-5 mg/L 2,4-D, 800 mg/L glutamine and different concentrations of sucrose. Cultures were kept in the dark at 28°C until enlargement, and callus formation were monitored. Observations of callus development and growth were made using Nikon light microscope and corresponding images of callus captured using Nikon digital sights DS-Fi1c camera (Nikon, 1300 Walt Whitman Road Melville, NY 11747-3064, USA). Different experiments were carried out to study the effect of anther density, sucrose concentration, and duration in culture on callus response.
Callus regeneration
To determine the frequency of callus formation following culture in the dark at 28˚C, the number of callus-bearing anthers were recorded. This best temperature shock pre-treatment (40˚C for 6 hours) was selected for pre-treatment. Callus was then transferred to the regeneration medium containing MS basal salts supplemented with 3% sucrose and phytagel 3-5% (w/v) and 1 mg/L 6-Benzyl amino purine (BAP) and 0.5 mg/L 1-Naphthalene acetic acid (NAA) for one month. Embryogenic callus formed were further cultured onto the same medium containing only 0.5 mg/L BAP at 1-month intervals until embryos were formed.
Histological analysis of callus derived from anthers
Anthers were sampled 2, 8, and 16 weeks after culture initiation. Histological analysis was performed on ten cultured anthers subjected to heat treatments. Twenty (20) anther-derived structures were sampled at different stages of culture ranging friable calli to globular embryos for the aim of observing embryogenic callus for evidences of various stages of differentiation. Thus, callus was sampled and fixed in Formalin-acetic acid-alcohol (FAA) (composed of 50% ethanol, 10% formaldehyde, and glacial acetic acid, 18:1:1) for 72 hours. The callus was then dehydrated through a series of graded ethanol of 30, 50, 70, 95, and 100% (v/v), followed by 100% butanol for 48 hours. Impregnation was followed by embedding the samples in Technovit 8100 resin (Heraeus Kulzer GmbH, Wehrheim, Germany).
Sample sections of 3.5-5.0μm thick were made using a rotary microtome (Histostat, Reichert Scientific Instruments) and thereafter were double stained with periodic acid Schiff’s reagent (Sigma-Aldrich) for starch- and protein-specific Naphthol Blue Black. All sections were studied under a light microscope (DM500, Leica GmbH, Wetzlar, Germany) and photographed with an attached camera (ICC50HD; Leica GmbH, Wetzlar, Germany). Observations for meristematic cells and vascular elements were using an inverted microscope (Nikon Alphaphot-2 YS2, Japan) equipped with a 100W mercury lamp and a UV light excitation as done previously by Kundu at al. 2014 [17].
Data collection and statistical analysis
Statistical analysis was performed using R-statistical package (R Core Team 2014) on five experiments consisting of five replicates per treatment. The data collected included the undeveloped anthers, swollen anthers, anthers producing callus and anthers producing embryos recorded for each Petri dish. Analysis of variance (ANOVA) was used to determine significant differences between responses in the treatments at P=0.05.
Androgenesis induction and development
At the time of inoculation, microspores were observable through a thin wall of anthers in NASE14 (Figure 1A) and NASE3 (Figure 1M). Cultured anthers for both genotypes became swollen in 28 days on MS culture medium and induced callus (Figure 1B and Figure 1N). Callus was observed forming inside the anthers (Figure 1D and Figure 1O). Anthers from both genotypes contained highly friable and fragile callus.
At 45 days of culture, callus development through the anther wall was observable (Figure 1F and 1G, Figure 1P and 1Q). At 45 days, embryogenic callus was also observed (Figure 1H and Figure 1S). Globular-embryos were induced from embryogenic callus after 20 days on MS medium containing BAP and NAA (Figure 1F). Torpedo embryos were also observed as shown in Figure 1I. A line of weakness on the anther wall open up and callus was coming from the inside separated lines (Figure 1P and Figure 1Q).
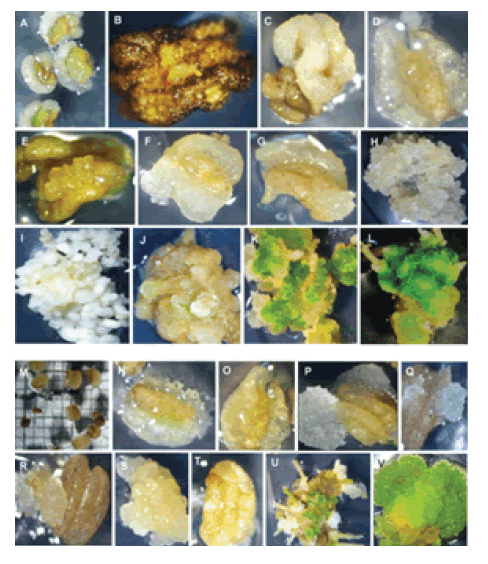
Figure 1: Callus induction and gametic embryogenesis of cassava genotypes (M. esculenta Crantz). NASE14 (A to L) and NASE3 (M to V). NASE14: (A) Fresh anthers on induction; (B) Callus wall dissolving exposing the microspores; (C) Bulging anthers on induction medium; (D) Anther becomes transparent exposing the callus inside the anther; (E) Callus proliferation emerging from the interior of the anthers; (F-G) Calli proliferation coming out from inside the anther; (G) Embryogenic callus on multiplication medium; (I) Globular embryos forming on MS basal salts medium; (H) Shoot formation; (J- L) Chlorophyll pigmentation in the secondary embryos after exposing to the light (556 μm); (L) Adventitious root development on the callus placed on rooting medium. NASE3: (M) Fresh anthers on induction; (N) Calli wall dissolving exposing the microspores; (O) Swollen anther and callus inside the anthers on induction medium; (P-Q) Different stages of callus proliferation emerging from the interior of the anthers; (R) Calli coming out from anther line of weakness; (S) Globular-embryos forming on MS basal salts medium; (T) Callus coming out from the anther wall; (V) Chlorophyll pigmentation in the secondary embryos after exposing to light at 556 μm; (U) Adventitious root development on the callus on rooting medium.
The anthers without heat treatment formed callus following culture. Callus proliferated, filling the petri dish on both liquid and solid medium within 30 days (data not shown). The highest percentage of enlarged anthers (43.75 ± 1.0) and callus formation (32.00 ± 0.9) was recorded in NASE14 on solid media (Table S1). However, on liquid medium, the highest percentage of enlarged anthers (35.50 ± 0.9) and callus formation rate (6.80 ± 1.0) was observed in NASE3 (Table S1).
The differences between media and genotypes were significant (P<0.05). Upon continuous presence of anthers in culture medium, the anther wall turned brown (Figure 1B). However, small globular structures emerged through ruptured anther wall (Figure 1T) and proliferated (Figure 1S) increasing in volume in the basal medium (Figure 1H). MS media containing 1mg/l BAP and 0.5mg/l NAA and 4% phytagel triggered compaction of the callus which led to induction of embryogenic callus (Figure 1U). Moreover in both genotypes, callus developed green spots on regeneration media under light conditions (Figure 1K-L and Figure 1V). However, conversion of the embryos into plants was not observed, under these culture conditions, although low frequency of roots (Figure 1L and Figure 1U) and abnormal shoot (Figure 1J) development were observed. For NASE3, dissolving of the anther wall was observed (Figure 1N), leading to exposure of the microspores in the liquid medium.
Callus production from cassava anthers under variable culture conditions
Callus production was significantly influenced (P<0.0001) by the sucrose concentrations 2, 9, 12, 15 and 20% (w/v), with genotype NASE3 having a higher mean callus production (75.0, 43.8, 29.6, 13.4 and 3.6) compared to NASE4 (65.0, 36.0, 26.0, 11.0 and 0.8) (Figure 2).
Similarly, there was a significant effect of genotype on callus production (P<0.005), showing greater callus production from NASE3 than NASE14. Effect of anther density on callus production shows that callus response decreased (P<0.0001) with increased anther density and at all anther densities NASE3 showed the higher response (P<0.05) than NASE14 in callus production (Figure 2B). Also, different incubation periods at 40˚C showed different responses (P<0.0001) with the highest production of callus observed at 6h incubation and lowest at 24h (Figure 2C).
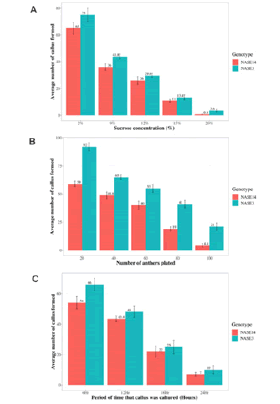
Figure 2. Effect of sucrose concentration (A), anther density (B), and period on culture (C) on callus formation, in cassava (M. esculenta) genotypes NASE14 and NASE3. Callus formation decreased with increasing concentration of sucrose, anther density and period on culture in both genotypes (A-C), and in all these cases, callus formation was consistently higher in NASE3 than NASE14.
Histology and embryogenesis of induced callus
Histological analysis of the cultured anthers revealed that embryogenic callus was formed inside the anthers (Figure 3). Vascular bundles from regenerating callus (Figure 3C) and differentiation of cells in embryogenic callus were observed (Figure 3D). Highly vacuolated undifferentiated cells were observed indicating a characteristic feature of friable callus (Figure 3B).
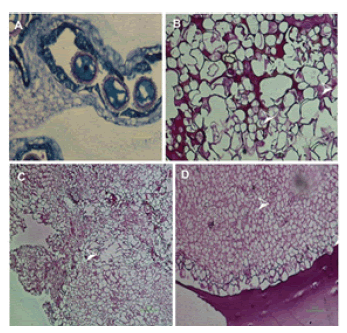
Figure 3. Histological assays of the cultured anthers. (A) Cultured microspore with the nucleus at the center, (B) Empty vacuoles of cells (white arrows), (C) Formation of the vascular bundles (white arrow) from regeneration callus, (D) differentiation of cells in the pro-embryo (white arrows).
In the cultured tetrads of both NASE3 and NASE14, the four microspores were morphologically similar (Figure 4A). At 7 days on medium, enlargement of the microspores in tetrads was observable (Figure 4E). Calli formation was observed in cultured microspores (Figure 4F-G) which later formed compact callus when transferred to solid medium (Figure 3H).

Figure 4.Microspore reprogramming in tetrads. (A) an immature tetrad, (B) embryogenesis in tetrads eight celled, (C-D) multi-cellular structures developing through the aperture area, (D) and (F-H) rapture of the callose wall, (I) Cali formation from the microspores, (J) Friable embryogenic callus.
The ability of pollen to redirect its development from gametogenesis to embryogenesis depends on the pollen’s stage of maturity at the time of initiation on culture. For cassava, the observable morphological trait that shows good correlation with the pollen development is a bud size of 2.3-2.6 mm [18]. In this study, small bud sizes targeting the tetrad stage of microspore development (2.0-2.8mm) were used, on the assumption that the size of anthers is correlated with microspore development stage. Anther pre-treatment is one of the factors needed to convert from the gametophytic pathway to embryogenesis [19]. Thus, in the current study, heat shock of anthers at 40°C for 6 hours was efficient to induce embryogenic callus. The anther wall was observed disintegrating and exposing the microspores. The callus was also observed to form within the anthers before they open indicating that the callus originated from the microspores and not the anther wall. Similar observations have been made in Salix integra [20]. Also, callus was observed to originate from within the anthers because pollen mother cells in the anther lobes underwent cell divisions to form a whitish mass [21].
When the embryogenic callus was cultured on regeneration medium, only greening of embryos was observed with tissue differentiation into vascular bundles, similar to previous observations by Perera et al. 2014 [22]. Regeneration of plants from embryogenic callus in cassava requires independent studies involving rigorous optimization of numerous parameters in culture although it seems more difficult to obtain regeneration from cells and meristems compared to more developed leaves and hypocotyls, possibly because of limited nutrients and hormone reserves. Sabbadini et al. 2019 [23] suggest that explants on growth media compete for a constant supply of water and nutrients and therefore reduce explant’s regeneration capacity. More efforts are needed to understand the factors that affect regeneration process in cassava. For instance, sometimes increasing explant density has been reported to increase shoot regeneration [24].
One other important factor in explant regeneration are the growth hormones especially cytokinins that play an important role in shoot organogenesis [25]. Accordingly, exogenous application of auxins is desirable to induce and enhance callusing response because auxins stimulate cell division, cell growth and tissue differentiation. In this study, 2,4-D at a concentration of 5 mg/L induced callus in anthers (data not shown) as has been shown in different crops such as in chickpea [26]. Furthermore, Abiri et al. 2017 [27] reported that applications of higher concentrations of 2,4-D (10 mg/L) significantly increased callus induction response in rice. However, in cassava, this concentration had no observable effect. A possible reason for the recalcitrance of cassava callusing is the formation of a thick exine wall Perera et al. 2013 [28] which lowers the penetration of the medium components into the microspore. Morphogenesis of androgenic callus, and subsequent differentiation are affected by the different plant hormones in the medium [29]. It is important to understand how morphogenesis in androgenic callus is affected at different media concentrations of cytokinins and auxins. Furthermore, the induction medium used is important for successful plant regeneration [28]. Ensuring that the callus selected and transferred to regeneration media is embryogenic is important to ensure green plant production. Therefore, further studies will put emphasis on identifying media required for production of embryogenic callus, as opposed to the non-embryogenic one [30,31].
Carbon sources show differential effect on callus induction using different explants [32,33]. In the current study, callus induction was highly influenced by the sucrose concentration and anther density with 2% sucrose and 20 anthers/plate anther density giving the best results. Sucrose concentration according to Warchoł et al. 2018 [34], is important in providing a source of carbon and energy in culture media, and keeping required osmotic conditions. Bogunia and Przywara 2000 [35] reported that higher concentrations of 8-12% is required for immature embryo development, while lower sucrose concentrations (2-3%) in the media is required for mature embryo regeneration [36].
We acknowledge the financial support provided by International Centre for Tropical Agriculture (CIAT) to conduct this work. We thank Mr. Kisekka Majidu (Makerere University, Uganda), for his help in the histology analysis, and Dr. Henry Wagaba (National Crops Resources Research Institute, Uganda) for helpful comments on an earlier version of this manuscript. We thank the two reviewers for their constructive comments that improved this manuscript.
Citation: Buttibwa M, Kawuki RS, Oshaba B, Eyokia M, Hershey C, Perera PIP, Heberle-Bors E, Baguma Y, Tugume AK (2020) In vitro Culture of Heat-treated Anthers Induces Embryogenic Callus in Cassava (Manihot esculenta Crantz). J Plant Biochem Physiol 8:249. DOI: 10.35248/2329-9029.20.8.249
Received: 03-Jun-2020 Accepted: 10-Jun-2020 Published: 17-Jun-2020 , DOI: 10.35248/2329-9029.20.8.249
Copyright: © 2020 Buttibwa M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.