Journal of Agricultural Science and Food Research
Open Access
ISSN: 2593-9173
ISSN: 2593-9173
Research Article - (2016) Volume 7, Issue 1
Endophytic bacteria residing within living plant tissues without substantially harming plants have found a large number of applications in today’s agriculture such as nutrient cycling, tolerance to biotic and abiotic stress as well as promotion of plant growth. Endophytic bacteria namely, Acetobacter and Azospirillum promised to be practically used in agriculture are nowadays thought of as most active components in association with cereals. So, in present study, endophytic bacteria mainly belonging to genera Acetobacter and Azospirillum were isolated from surface sterilized plant parts of species Cynodon dactylon (Durva), Pothos scandens (Money plant), Ipomea batata (Sweet potato), Saccharum officinarum (Sugarcane) cv. CO.LK-8001 and CO.-84135, Musa paradica (Banana) and Zea mays (maize) cv. GM-6 by using LGIP and Nitrogen free bromothymol blue media selective for growth of Acetobacter and Azospirillum, respectively. Inoculation of maize cv. GM-3 with endophytic bacterial isolates in combination with half and full Recommended dose (RD) of urea recorded significant increase in all the growth parameters wherein A-9 was found the best for growth stimulation as compared to other treatments.
<Keywords: Endophytic; Acetobacter; Azospirillum; Maize
It is well known that “corn” crop is considered among the most important cereal crops either all over the world that consumes huge quantities of chemical nitrogenous fertilizers (16 million tones/year). Many attempts have been tried to replace a part of those harmful chemical fertilizers by bio fertilizers to get yield of a good quality without loss in its quantity. Bio fertilizers are the microbial inoculants which help in increasing crop productivity by way of biological nitrogen fixation, increased availability or uptake of nutrients through solubilization or increased absorption, stimulation of plant growth through hormonal action or antibiosis, earlier days the term biological nitrogen fixation (BNF) was restricted to the endophytic bacteria of genus Rhizobia which can form mutually beneficial symbiotic nitrogen fixing association with leguminous plants. But now recent researches have intensely brought down the difficulties and increased the scope for nitrogen fixation in cereals by means of endophytic nitrogen fixation [1]. Kado defined endophytic bacteria that reside within plant tissue without doing substantial harm or gaining benefit other than securing residency. Among endophytic bacteria Acetobacter is interesting because it carries out nitrogen fixation under aerobic conditions, because it requires oxygen for the production of large quantities of ATP required for nitrogen fixation. A. diazotrophicus is of special interest because it can excrete almost half of the fixed nitrogen in the form potentially available to plants. Azospirillum, an associative microaerophilic organism can live in association with diverse group of plants. Associative nitrogen fixation, capability to produce plant growth promoting antifungal / antibacterial substances and their effect on root morphology are the principal mechanisms responsible for the observed promotion of crop yield. Inoculation with Azospirillum results in enhanced assimilation of mineral nutrients (N, P, K, Fe2+), and water and offers resistance to pathogens. The utilization of inoculants containing biofertilizer is becoming more popular due to increasing reports of expressive gains in grain yields. Many workers have proved the role of biofertilizers in reducing nitrogen fertilizer requirement of the corn crop. Fukami et al. [2] reported in field experiments that inoculation with A. brasilense allowed for a 25% reduction in the need for N fertilizers. So, present investigation was carried out with an objective to find out effective native biofertilizer strains to reduce chemical nitrogen fertilizer input in maize crop.
Source of isolates
In present study, isolation of endophytic bacteria was attempted from various plant parts (root, stem and leaves) of species viz. Cynodon dactylon (Durva), Pothos scandens (Money plant), Ipomea batata (Sweet potato), Saccharum officinarum (Sugarcane) cv. CO.LK-8001 and CO.- 84135, Musa paradica (Banana) and Zea mays (maize) cv. GM-6.
Isolation procedure for endophytic bacteria
For isolation, enrichment culture technique was employed. After collection, the plant was thoroughly washed with tap water to remove any soil particles adhered to it and was cut in to small pieces approximately of 0.5 cm size and surface sterilized by treatment of 1% HgCl2 (Mercuric chloride) followed by treatment of 70% ethyl alcohol and finally washing with sterile distilled water. These surface sterilized pieces were then slightly burned in the low flame of burner to alleviate any surface adhering microorganism and then inoculated in to sterilized LGIP and NFB medium [3] (Cavalcante and Dobereiner), selective for growth of Acetobacter and Azospirillum, and incubated at 28 ± 2°C for one week on shaker to ensure good growth of organisms. After giving two successive transfers in same selective medium, 100 μl aliquot was inoculated in semisolid LGIP and NFB media to check pellicle formation. Pellicles forming bacterial cultures were streaked over the surface of solid LGIP and NFB medium to obtain colonial growth of organism. Single colony with typical morphology of Acetobacter and Azospirillum as described by Dobereiner [4] were picked from these plates to subculture by restreaking onto LGIP and NFB plates and used for further study (Table 1).
| S No | Name of isolate | Source of organism | ||
|---|---|---|---|---|
| Scientific name | Common name | Plant part | ||
| 1. | A-1 | Pothos scandens | Money plant | Leaf |
| 2. | A-2 | Cynodon dactylon | Durva | Leaf |
| 3. | A-3 | Ipomea batata | Sweet potato | Root |
| 4. | A-4 | Saccharum officinarum cv. CO.-84135 | Sugarcane | Stem |
| 5 | ACG-1 | Acetobacter diazotrophicus from Saccharum officinarum | Sugarcane | Stem |
| 6. | A-6 | Pothos scandens | Money plant | Leaf |
| 7. | A-7 | Cynodon dactylon | Durva | Leaf |
| 8. | A-8 | Musa paradica | Banana | Root |
| 9. | A-9 | Saccharum officinarum cv. CO.LK-8001 | Sugarcane | Root |
| 10 | ASA-1 | Azospirillum lipoferum from Pennisetum glucam | Bajara | Root |
Table 1: Endophytic bacterial isolates of different plant parts and species.
In vitro efficacy testing of endophytic bacterial isolates on maize cv. GM-3
In vitro efficacy of isolates on maize seeds was tested on solid water agar in petri plates. Maize seeds of variety GM-3 were surface sterilized by treatment of 0.1% HgCl2 solution 3 times for time interval of 15 mins followed by treatment with ethyl alcohol and finally washing with sterile distilled water. These surface sterilized seed were then inoculated with 0.01 ml. of previously grown starter cultures of Acetobacter and Azospirillum isolates in LGIP and NFB broth and allowed to stand for 30 mins. Control seeds without treatment were also used as check. Five treated seeds were now allowed to grow on petri plates containing sterilized 1% water agar medium under dark conditions. After one week of incubation the plantlets were removed carefully from water agar and root length, shoot length, fresh weight and dry weight were measured.
In vivo efficacy of endophytic bacterial isolates on maize cv. GM-3
Characteristics of soil used for pot trial: The soil of the experimental pot was sandy loam, locally known as “Goradu”. The soil was well drained and retentive of moisture. It responded well to irrigation and manuring and was reasonably suitable for maize cultivation. Ultimate Physico-chemical condition of the experimental soil was analyzed at Department of Soil Science, BACA, AAU, Anand is given in Table 2.
| S No | Element | Properties of soil |
|---|---|---|
| 1. | Total nitrogen (%) from soil | 0.021 |
| 2. | Organic Carbon (%) | 0.24 |
| 3. | Available P2O5 (kg ha-1) | 22.78 |
| 4. | Available K2O (kg ha-1) | 176.88 |
| 5. | Electrical Conductivity (dSm-1 at 25°C) | 0.14 |
| 6. | pH | 8.41 |
Table 2: Chemical characteristic of experimental soil.
Seed inoculation efficacy of endophytic bacterial isolates on maize cv. GM-3: This trial was undertaken with graded doses of urea i.e., recommended dose (120 kg/ha) and half of the recommended dose (60 kg/ha) for demonstration of nitrogen savings in maize which is highly exhaustive crop. GM-3 seeds were inoculated with previously grown starter cultures of Acetobacter and Azospirillum isolates in LGIP and NFB broth having 108-109 bacterial counts at the rate of 5 ml/kg seeds and allowed to stand for 30 mins. Five seeds were sown at the depth of 5 cm in pots having 10 kg soil collected from agronomy farm, AAU, Anand. Treatments were set as per point mentioned in treatment details. Agronomic practices were common for all the treatments. Factorial completely Randomized Design (F-CRD) was applied with 22 treatments and 3 replications (Table 3).
| Tr. No. | Nitrogen (N) | Bacterial isolate (B) | ||
|---|---|---|---|---|
| T1 | N1 | 60 kg/ha (½ RD) | B0 | No biofertilizer (control) |
| T2 | B1 | A-1 | ||
| T3 | B2 | A-2 | ||
| T4 | B3 | A-3 | ||
| T5 | B4 | A-4 | ||
| T6 | B5 | ACG-1 | ||
| T7 | B6 | A-6 | ||
| T8 | B7 | A-7 | ||
| T9 | B8 | A-8 | ||
| T10 | B9 | A-9 | ||
| T11 | B10 | ASA-1 | ||
| T12 | N2 | 120 kg/ha (RD) | B0 | No biofertilizer (control) |
| T13 | B1 | A-1 | ||
| T14 | B2 | A-2 | ||
| T15 | B3 | A-3 | ||
| T16 | B4 | A-4 | ||
| T17 | B5 | ACG-1 | ||
| T18 | B6 | A-6 | ||
| T19 | B7 | A-7 | ||
| T20 | B8 | A-8 | ||
| T21 | B9 | A-9 | ||
| T22 | B10 | ASA-1 |
Note: RD=Recommended dose
Table 3: Treatment details.
Plant height at 30 DAS, 60 DAS and 90 DAS
At the time of 30, 60 and 90 days after sowing, plant height of five plants was measured and average was reported as plant height.
Stem girth and internode length at 60 DAS
At the time of 60 days after sowing, internode length and stem girth of five plants were measured and average was reported as internode length and stem girth.
Fresh and dry shoot and root weight
After harvesting fresh shoot and root weight of 5 individual excised plants was recorded and their average was reported as fresh shoot and root weight. Shoots and roots were dried under natural conditions and weight of 5 individual plants was recorded and their average was reported as dry shoot and root weight.
Chemical analysis of soil
Organic carbon (%) by Walkley and Black’s titration method: 1.0 gm of the soil sample (finely ground to pass through 0.5 mm. sieve) was taken into a 500 ml Erlenmeyer flask. To this, 10 ml of normal potassium dichromate solution was added, followed by 20 ml. of conc. H2SO4. This mixture was shaken for half an hour. At the end of the period, 200 ml of water was added to the flask with the addition of 10 ml 85% H3PO4 and 10 ml of the indicator diphenylamine. When the contents of the flask attain a dark blue color, titration was done against 0.5 N ferrous sulphate solution till the contents attain a brilliant green color.
Calculation:
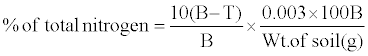
% of total nitrogen=% OC × 0.0862
Acetobacter and Azospirillum soil counts: Soil samples were collected before sowing and at the time of harvest pot wise separately and stored in polythene bags and kept in refrigerator till processed. Acetobacter and Azospirillum counts were done by taking 1 gm soil sample in sterile 100 ml D/W and shaken it for 1 hour and 0.1 ml sample was taken aseptically from it and transferred in 4.5 ml D/W containing dilution tube to make up to 10-8 dilutions by serial dilution method and spreaded on LGIP and N free BTB agar plates and incubated for 48 hours. After 48 hours, counts were taken by calculating cfu/gm.
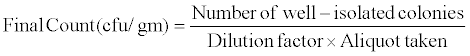
Source of isolates
In all, total 24 strains were isolated from different plant parts and species. Out of these, total 10 isolates were selected on the basis of their appearance and vigor to grow on NFB (5-isolates) and LGIP (5-isolates) medium. Standard strains of Azospirillum lipoferum (ASA-1) and Acetobacter diazotrophicus (ACG-1) were collected from Department of Agriculture Microbiology, BA College of Agriculture, Anand Agricultural University, Anand and used as positive check during entire investigation. After getting pure culture of organism series of morphological, physiological and biochemical tests were carried out and which have well established similarities of isolates A-1 to A-5 with genus Acetobacter and isolates A-6 to A-10 with genus Azospirillum So, we can classify them according to [5] Bergey’s manual of systematic bacteriology as Acetobacter and Azospirillum species.
In vitro efficacy testing of endophytic bacterial isolates on maize cv. GM-3
Inoculation with all the bacterial strains had significant effect on the development of maize cv. GM-3 after 14 days of inoculation. Isolate A-9 showed significantly higher root (15.8 cm) and shoot length (13.2 cm), fresh (0.9 g) and dry biomass (0.5 g) which was at par with isolate A-7 and ASA-1 except shoot length (Table 4). All the bacterial inoculants showed maximum root hairs (Figure 1) as compared to noninoculated control.
| Treatment | Root length(cm) | Shoot length(cm) | Fresh biomass weight(g) | Dry biomass weight(g) |
|---|---|---|---|---|
| Control | 6.7f | 6.5fg | 0.5d | 0.1d |
| A-1 | 13.2bc | 10.9b | 0.9a | 0.5a |
| A-2 | 8.4e | 10.9b | 0.7c | 0.4abc |
| A-3 | 13.3bc | 5.6g | 0.7c | 0.3c |
| A-4 | 12.4cd | 8.7cd | 0.7bc | 0.3c |
| ACG-1 | 11.1d | 7.5ef | 0.6c | 0.3c |
| A-6 | 13.3bc | 9.7bc | 0.7c | 0.3c |
| A-7 | 14.8ab | 10.5b | 0.9a | 0.4bc |
| A-8 | 13.7bc | 7.9de | 0.8ab | 0.5ab |
| A-9 | 15.8a | 13.2a | 0.9a | 0.5a |
| ASA-1 | 14.8ab | 10.8b | 0.8ab | 0.5ab |
| SEM | 0.5 | 0.4 | 0.03 | 0.03 |
| CD at 5 % | 1.4 | 1.1 | 0.1 | 0.1 |
| CV % | 6.6 | 6.9 | 7.8 | 16.0 |
Note: Treatment means with the letter/letters in common are not significant by Duncan’s New Multiple Range Test at 5% level of significance.
Table 4: In vitro efficacy testing of isolates on maize cv. GM-3.
Seed inoculation efficacy of Acetobacter and Azospirillum isolates on maize cv. GM-3
A pot trial was carried out with recommended dose (120 kg N/ha) and half of the recommended dose (60 kg N/ha) of urea to study the effect of various endophytic bacterial isolates on maize cv. GM-3 and also to demonstrate nitrogen savings in maize due to bio-inoculants.
Effect on plant height, internode length and stem girth
Data regarding changes in the plant height at 30, 60 and 90 DAS and internode length and stem girth at 60 DAS as well as root and shoot fresh and dry weight due to inoculation of endophytic bacteria along with recommended and half of the recommended doses of nitrogen are presented in Table 5. The results revealed that seed inoculation of all the isolates significantly influenced plant height at 30, 60 and 90 DAS (Figures 2 and 3). Among the different strains of endophytic bacteria isolate A-9 recorded maximum plant height (65.0, 87.8 and 116.9 cm) at 30, 60 and 90 DAS respectively which was significantly superior over uninoculated control (51.9, 64.0 and 66.8 cm). Application of urea significantly influenced plant height. Treatment with R.D. gave 59.2, 81.2 and 106.1 cm. average plant height at 30, 60 and 90 DAS which was significantly superior over half R.D. (56.7, 76.1 and 93.8 cm). However, interaction between endophytic bacteria and doses of nitrogen proved to be non-significant at 30, 60 and 90 DAS suggesting that at any level of nitrogenous fertilizer bacterial inoculation improves growth of plant. Swedrzynska et al. [6] reported that maize (Zea mays sp. Saccharata L.) inoculated with Azospirillum brasilense showed 27% increase in yield and higher cob mass than uninoculated control under different cultivation conditions. Riggs et al. [7] conducted greenhouse experiment in maize without N fertilizer. Inoculation of G. diazotrophicus Pal-5 significantly increased dry weight of maize genotypes Mo17, B14 and B84 by 42.6, 25.2 and 15.6 percentage, respectively. In field trail where N @ 224 kg/ha was applied, maize genotypes B73xMo17, 36H36 and 3905 showed increase in yield by 25.3, 23.4 and 14.4 percentage, respectively. Moreover, the results also revealed that seed inoculation of all the isolates significantly influenced inter node length at 60 DAS. Among the different strains of endophytic bacteria isolate A-8 recorded highest inter node length (8.5 cm) 60 DAS which was significantly superior over uninoculated control (6.5 cm), closely followed by isolate A-3 (8.4 cm) and A-9 (8.1 cm). Similarly application of endophytic bacterial cultures with half R.D. and full R.D. significantly influenced stem girth at 60 DAS. Isolate A-9 reported significantly higher stem girth (5.2 cm) as compared to control (4.2 cm). The data also indicated that stem girth per plant also significantly influenced due to application of urea. N2: 120 kg/ha recorded higher stem girth (4.9 cm). Here, interaction between endophytic bacterial inoculants with and without urea also significantly affected stem girth of maize at 60 DAS. These findings are confirmed by Osmar et al. [8] who had reported maize Cargil - 909 seeds inoculated with Azospirillum sp. RAM-7 and RAM-5 strains can reduce 40% of the recommended N fertilizer under field conditions. Mehnaz et al. [9] studied effect of G. azotocaptan strain DS1 isolated from maize rhizosphere and G. diazotrophicus strain Pal-5 and nif D mutant strain on different maize cultivars and reported that G. diazotrophicus Pal- 5 and G. azotocaptan DS-1 were resulted in significant increase in shoot weight of corn variety 39D82 and 39M27, respectively in sand experiment. G. azotocaptan DS-1 significantly increased root weight of corn variety 39H84 and G. diazotrophicus nif D significantly increased shoot weight of corn variety 39M27 soil experiment.
| Treatment | Plant height (cm) | Internode length (cm) | Stem girth (cm) | Root fresh weight (g) | Root dry weight (g) | Shoot fresh weight (g) | Shoot dry weight (g) | ||
|---|---|---|---|---|---|---|---|---|---|
| 30 DAS | 60 DAS | 90 DAS | 60 DAS | 90 DAS | |||||
| N levels | |||||||||
| N1 (60 kg/ha) | 56.7 | 76.1 | 93.8 | 6.9 | 4.5 | 94.7 | 60.3 | 81.5 | 32.1 |
| N2 (120 kg/ha) | 59.2 | 81.2 | 106.1 | 9.5 | 4.9 | 108.3 | 70.7 | 89.2 | 34.9 |
| SEM | 0.55 | 0.9 | 2.1 | 0.2 | 0.1 | 3.0 | 1.2 | 0.9 | 1.1 |
| C.D. at 5% | 1.57 | 2.6 | 6.1 | 0.6 | 0.1 | 8.6 | 3.3 | 2.6 | 3.1 |
| B levels | |||||||||
| B0: No biofertilizer | 51.9 | 64.0 | 66.8 | 6.5 | 4.3 | 61.0 | 32.4 | 74.1 | 26.1 |
| B1 : A-1 | 57.1 | 78.6 | 96.8 | 7.7 | 4.6 | 79.6 | 45.8 | 82.4 | 29.7 |
| B2 : A-2 | 58.9 | 82.2 | 98.6 | 7.1 | 4.7 | 77.2 | 43.3 | 88.0 | 29.9 |
| B3: A-3 | 61.0 | 84.7 | 104.8 | 8.4 | 4.9 | 126.0 | 85.7 | 89.6 | 39.0 |
| B4: A-4 | 56.0 | 80.0 | 98.5 | 7.1 | 5.1 | 93.7 | 62.0 | 84.1 | 26.5 |
| B5: ACG-1 | 55.3 | 69.3 | 94.9 | 6.0 | 4.5 | 98.1 | 64.3 | 78.2 | 31.5 |
| B6: A-6 | 53.0 | 75.7 | 101.2 | 7.7 | 4.4 | 119.0 | 82.9 | 85.9 | 36.2 |
| B7: A-7 | 55.0 | 84.7 | 102.2 | 8.0 | 4.9 | 90.8 | 53.8 | 90.3 | 37.4 |
| B8: A-8 | 62.5 | 84.1 | 114.7 | 8.5 | 4.6 | 127.3 | 87.5 | 87.0 | 37.1 |
| B9: A-9 | 65.0 | 87.8 | 116.9 | 8.1 | 5.2 | 131.5 | 88.3 | 98.5 | 43.2 |
| B10: ASA-1 | 61.3 | 74.5 | 104.0 | 8.0 | 4.9 | 112.0 | 74.5 | 83.4 | 31.9 |
| SEM | 1.3 | 2.3 | 5.0 | 0.2 | 0.12 | 7.1 | 2.7 | 2.1 | 2.6 |
| C.D. at 5% | 3.7 | 4.5 | 14.2 | 0.6 | 0.34 | 20.1 | 7.7 | 6.0 | 7.3 |
| Interaction effect | |||||||||
| N × B | NS | NS | NS | NS | 0.48 | NS | NS | NS | NS |
| CV % | 5.5 | 7.1 | 12.2 | 16.1 | 6.2 | 17.0 | 10.1 | 6.0 | 18.7 |
Table 5: Effect on plant height, internode length and stem girth.
Effect on root and shoot fresh and dry weight at harvesting
Data regarding changes in the root and shoot fresh and dry weight at 90 DAS are presented in Table 5. Among different strains of endophytic bacteria isolate A-9 recorded highest root fresh weight (98.5 g) and root dry weight (43.2 g), shoot fresh weight (131.5 g) and shoot dry weight (88.3 g), as compared to uninoculated control, closely followed by A-8, A-3 and A-6 (Figure 4). Interaction between endophytic bacteria and doses of urea proved to be non-significant for root and shoot fresh and dry weight at 90 DAS. Overall, from the above results it is ascertained that bacterial inoculation had significant impact on growth and growth attributes of maize cv. GM-3 and can substitute about 50% nitrogen fertilizer in maize crop (Figure 5). These results are in conformity with Cohen et al. [10] who had reported that maize (Zea mays) plants inoculated with A. brasilense Sp-7 and CD-1 significantly increased dry weight of plants (i.e., 26 and 20% respectively) after 4 weeks of inoculation as compared to uninoculated control.
Effect on soil nutrient content and bacterial counts
Data regarding changes in soil organic carbon, total nitrogen and soil count at 90 DAS are presented in Table 6. Data revealed that T22 recorded maximum O.C. (0.317%) and total nitrogen content (0.027%) as compared to T1 (0.292% O.C. and 0.025% N) and T12 (0.238% O.C. and 0.020% N). T16 recorded highest Acetobacter counts (7.7 × 104 cfu/g) and T20 recorded highest Azospirillum counts (7.5 × 104 cfu/g) and making soil fertile for further cultivation. Above results, indicates that application of endophytic bacteria by seed treatment not only improves the growth of treated crop but also benefits the subsequent crop to be taken by improving soil nutrient content and bacterial counts. These findings are in agreement with those of Das and Saha [11] who also reported that Azotobacter and Azospirillum alone and in combination with recommended dose of N increased total Nitrogen content in soil. Rao and Charyulu [12] reported that A. brasilense inoculation +40 kg N ha-1 gave Total N 3.94 mg N/g as compared to control (2.98 mg N/g) in foxtail millet. Smith et al. reported that A. brasilense inoculation showed Total N 1.34 mg N/g as compared to control (1.2 mg N/g) in sorghum. Overall results indicated that all the isolates noticeably increased root and shoot length, fresh and dry biomass of maize cv. GM-3 in laboratory. In a pot efficacy testing, all the bacterial treatments have eye catching impact on growth and growth attributes of maize cv. GM-3. Isolate A-9 recorded significantly higher plant growth parameters under pot trial condition.
| Treatment | % O.C. | % N | Acetobacter counts (cfu/g) | Azospirillum counts (cfu/g) |
|---|---|---|---|---|
| T1 | 0.292 | 0.025 | 2.6 × 103 | 4.8 × 103 |
| T2 | 0.250 | 0.022 | 5.5 × 104 | - |
| T3 | 0.216 | 0.019 | 6.8 × 104 | -- |
| T4 | 0.291 | 0.025 | 5.8 × 104 | - |
| T5 | 0.195 | 0.017 | 3.9 × 104 | - |
| T6 | 0.294 | 0.025 | 4.8 × 104 | - |
| T7 | 0.217 | 0.019 | 5.5 × 104 | |
| T8 | 0.302 | 0.026 | 4.0 × 104 | |
| T9 | 0.299 | 0.026 | 5.0 × 104 | |
| T10 | 0.217 | 0.019 | 6.8 × 104 | |
| T11 | 0.214 | 0.018 | 4.1 × 104 | |
| T12 | 0.238 | 0.020 | 1.8 × 104 | 4.0 × 104 |
| T13 | 0.315 | 0.027 | 6.7 × 104 | - |
| T14 | 0.291 | 0.025 | 4.0 × 104 | - |
| T15 | 0.317 | 0.027 | 5.0 × 104 | - |
| T16 | 0.215 | 0.019 | 7.7 × 104 | - |
| T17 | 0.220 | 0.019 | 5.2 × 104 | - |
| T18 | 0.210 | 0.018 | - | 7.5 × 104 |
| T19 | 0.313 | 0.027 | - | 5.0 × 104 |
| T20 | 0.215 | 0.018 | - | 6.8 × 104 |
| T21 | 0.165 | 0.014 | - | 5.2 × 104 |
| T22 | 0.317 | 0.027 | - | 6.3 × 104 |
| Before | 0.240 | 0.021 | 2.1 × 103 | 4.3 × 103 |
Table 6: Effect on soil organic carbon, nitrogen and bacterial counts.