
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2022)
UC-II® is a unique joint health ingredient derived from chicken sternum. In a previous study, UC-II® improved knee extension range of motion and extend exercise time before the onset of knee pain in healthy participants. The current exploratory post hoc analyses sought to assess items from the Knee Injury and Osteoarthritis Outcome Score (KOOS) in healthy participants aged ≥ 50 years who previously participated in a randomized controlled trial. All participants reported knee pain following a standardized stepmill test and were randomized to receive placebo or 40 mg UC-II® supplementation containing ≥ 3% (≥ 1.2 mg) undenatured type II collagen for 120 days. For the current post hoc analyses, 17 participants (UC-II® =9, placebo=8) met the acceptance criteria (age ≥ 50 years). Analysis was carried out for the KOOS survey results with modified Intent-To-Treat Analysis (mITT). P-values ≤ 0.05 were considered statistically significant. After 120 days of supplementation, participants in the UC-II® supplementation group exhibited statistically significant improvements versus the placebo in select KOOS items including reduced pain during going up or down stairs, decreased discomfort in climbing ascending stairs or bending to floor to pick an object, or squatting during physical activity (p<0.05). UC-II® supplementation has the potential to improve knee joint function, mobility, flexibility, free movements and performance of daily activities in healthy participants ≥ 50 years old with exercise-induced knee pain.
Knee pain; Undenatured type II collagen; Physical function; Joint mobility; Knee joint function
Physical exercise elicits many benefits when performed in a consistent and progressive manner. Indeed, research has shown exercise to improve multiple factors of health, including joint health and function [1], blood pressure [2], glucose disposal [3], body composition [4], and even cognitive function [5]. However, barriers to exercise are plentiful and multifactorial. One such barrier is knee pain, adults with knee pain report lower levels of physical activity and possess uncertainty about their ability to exercise with knee pain [6].
Multiple potential remedies exist to reduce knee pain during exercise, including both activity therapies [1,7] as well as nutraceutical therapies [8]. One such nutraceutical therapy that has shown promise is supplementation with UC-II® , a unique undenatured type II collagen supplement derived from chicken sternum. Previous research has shown undenatured type II collagen supplementation to improve knee extension range of motion in healthy individuals with exercise-induced knee pain [9]. Similar benefits have been reported in animal studies, with both arthritic dogs [10] and arthritic horses [11] showing reduced pain during joint manipulation following supplementation with undenatured type II collagen.
UC-II® collagen type II is a naturally occurring ingredient that contains a glycosylated, undenatured type II collagen [12]. Type II collagen is the primary constituent of articular cartilage, which protects against wear and tear associated with joint mobilization and movement. The collagen fiber network fuses with a hydrated proteoglycan matrix to provide protection and cushioning in the joint [13]. Maintaining the integrity of collagen type II fiber matrix is of significant interest to fitness enthusiasts as a loss of cartilage homeostasis can disrupt the collagen-proteoglycan matrix, thus exacerbating knee pain and potentially leading to osteoarthritis [14].
Since knee pain, and joint pain in general, can present as self- diagnosed contraindications to exercise, managing and reducing joint pain is a common step for improving exercise adherence in multiple populations. Previous work by Lugo, et al. [9] has shown that consuming 40 mg/day of UC-II® collagen type II (providing ≥ 3% undenatured type II collagen) for 120-days improved knee range of motion and reduced pain during exercise in healthy participants aged 46.1 ± 1.5 years. In addition to assessing multiple indices of knee function and pain during exercise, Lugo and colleagues [9] administered the Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaire to study participants. The KOOS survey is a validated assessment for individual responses to questions encompassing five domains:
1. Pain;
2. Other symptoms;
3. Function in daily living;
4. Function in sport and recreation and
5. Knee-related quality of life.
The KOOS survey is useful for assessing changes in knee pathology over time, with or without treatment.
Previously, no significant differences were noted in KOOS survey outcomes between UC-II® supplementation and placebo study groups consisting of healthy participants aged 30 to 65 years with reported knee pain [9]. However, it is known that older individuals typically report greater incidence of knee pain than younger individuals, even in >55 age group [15]. Therefore, in this study, a sub-analysis was undertaken to determine whether or not, participants ≥ 50 years old garnered further benefit from UC- II® supplementation over placebo as measured by KOOS survey scores. It was hypothesized that individuals ≥ 50 years old would experience additional benefits of UC-II® supplementation which would be shown in KOOS survey scores relative to a placebo.
Study design
This article presents an exploratory post hoc analysis of a previously published randomized, double-blind, placebo-controlled study investigating UC-II(R) collagen's effect on improving knee joint function [9]. In this prior study (Figure 1), 55 of 106 screened participants met the eligibility criteria [9]. Only healthy adults who presented with no knee joint pain at rest and no diagnosable markers indicative of active arthritic disease, as outlined by the American College of Rheumatology (ACR) guidelines [16], were admitted into the study [9]. Herein, an analysis was conducted on data collected from the KOOS knee survey at baseline (Day 0) and final post visit (Day 120) in only the participants who were greater than or equal to 50 years of age. The KOOS survey is a validated instrument consisting of 42 questions [17]. KOOS measures subjective information on their knees and their ability to perform daily activities from the past week. Applying this age criteria (≥ 50 years old) to the 55 previously eligible participants yielded 17 total participants, 9 for UC-II® and 8 for placebo. Demographic and descriptive data are provided in Table 1 below. The investigational study product, UC- II® , is derived from chicken sternum. It is manufactured using a patented, low temperature process to preserve its native structure. For the clinical study, 40 mg of UC-II® material providing ≥ 3% (≥ 1.2 mg) of undenatured type II collagen, was encapsulated in an opaque capsule with excipients and visually identical placebo capsule was administered [9]. The study protocol was approved by an external institutional review board (IRB) and the study agreed with the Declaration of Helsinki (version 1996). Prior to engaging in study protocols, each participant voluntarily signed an informed consent form approved by the IRB.
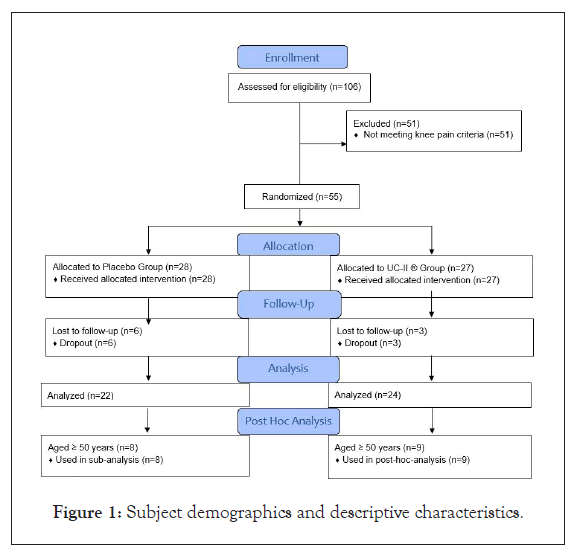
Figure 1: Subject demographics and descriptive characteristics.
Statistical analysis
The results were statistically analyzed using methods of descriptive and inferential statistics. The modified intent-to-treat method was used for the analysis. The pre/post differences were compared between the groups using an analysis of covariance model with the baseline value as the covariate. P<0.05 was determined as statistical significance.
An original dataset composed of 55 participants [9] was filtered to include only participants aged ≥ 50 years. This filter yielded 17 total participants, 9 in the UC-II® group and 8 in placebo. Both groups included 2 male participants while the UC-II® group and placebo group were comprised of 7 and 6 females, respectively. Age, physical stature, and resting vitals were not statistically different between groups (Table 1).
| Variable | UC-II® (n=9) | Placebo (n=8) |
|---|---|---|
| Age (yr.) | 53.8 ± 3.4 | 58.4 ± 4.6 |
| Ethnicity-n (%) | ||
| White | 2 (22.2) | 3 (37.5) |
| Asian | 1 (11.1) | 2 (25.0) |
| African American | 0 ( 0.0) | 0 ( 0.0) |
| Hispanic | 6 (66.7) | 2 (25.0) |
| American Indian/Alaska Native | 0 ( 0.0) | 0 ( 0.0) |
| Hawaiian/Pacific Islander | 0 ( 0.0) | 0 ( 0.0) |
| Other | 0 ( 0.0) | 0 ( 0.0) |
| White/Hispanic | 0 ( 0.0) | 1 (12.5) |
| Gender-n (%) | ||
| Male | 2 (22.2) | 2 (25.0) |
| Female | 7 (77.8) | 6 (75.0) |
| Temperature | 98.3 ± 0.4 | 98.2 ± 0.7 |
| Systolic blood pressure (SBP) | 113 ± 11.9 | 122 ± 14.1 |
| Diastolic blood pressure (DBP) | 71.2 ± 7.3 | 73.4 ± 9.2 |
| Pulse rate (bpm) | 70.4 ± 7.6 | 67.6 ± 8.5 |
| Respiration rate | 15.6 ± 1.9 | 15.1 ± 1.2 |
| Weight (kg) | 65.8 ± 8.1 | 66.2 ± 7.7 |
| Height (cm) | 161.8 ± 6.9 | 162.1 ± 8.6 |
| BMI (kg/m2) | 24.8 ± 3.2 | 25.3 ± 2.3 |
Note: Ethnicity and Gender is reported as n (% n). All other variables are mean ± SD
Table 1: Subject demographics and descriptive characteristics.
After 120 days of supplementation, participants in the UC-II® group exhibited a statistically significant improvement versus placebo in KOOS items which include reduced pain during going up or down stairs, decreased discomfort in climbing ascending stairs, bending to floor to pick an object, and squatting during period of high physical activity (p<0.05, Table 2).
| KOOS Parameter | Placebo (n=8) | UC-II® (n=9) | P value | ||
|---|---|---|---|---|---|
| BL | Day 120 | BL | Day 120 | ||
| Going up or down stairs | 3.375 ± 0.52 | 2.875 ± 0.64 | 3.222 ± 1.09 | 2.111 ± 0.60 | 0.0289* |
| Standing upright | 2.000 ± 0.93 | 2.000 ± 0.93 | 2.556 ± 0.53 | 1.556 ± 0.53 | 0.0522 |
| Ascending stairs | 3.125 ± 0.84 | 3.125± 0.99 | 2.889 ± 1.36 | 2.000 ± 0.87 | 0.0328* |
| Bending to floor/pick up an object | 2.875 ± 0.64 | 3.125 ± 0.99 | 2.556 ± 1.01 | 2.222 ± 0.44 | 0.0413* |
| Squatting | 3.125 ± 0.99 | 3.125 ± 0.84 | 3.222 ± 0.97 | 2.111 ± 0.78 | 0.0250* |
| How much are you troubled with lack of confidence in your knee? | 3.375 ± 0.92 | 2.875 ± 0.84 | 2.333 ± 0.5 | 1.778 ± 0.44 | 0.0196* |
Note: Data mean ± SD. *p<0.05 shows significant difference between UC-II and placebo group at Day 120; The Knee Injury and Osteoarthritis Outcome Score (KOOS).
Table 2: KOOS individual physical functions score for placebo and UC-II® groups at baseline (Day 0) and final visit (Day 120).
This study endeavored to investigate the effects of 120-days of undenatured type II collagen supplementation on KOOS survey scores in participants at least 50 years of age or above with exercise-induced knee pain. The primary rationale for investigating this age group stems from prior knowledge that knee pain is known to increase with advancing age [15]. The findings support the study hypothesis as lower self-report pain, per the select KOOS survey items, was apparent in the undenatured type II collagen supplementation group over placebo at Day 120 of supplementation.
Prior research indicated that undenatured type II collagen can increase anti-inflammatory cytokines (IL-4, TGF-β) and decreases circulating levels of inflammatory cytokines (IL-2, IL-17) [18]. This combination can acutely lower the incidence of arthritic symptoms. The ability to modify the immune response after consumption of a food-based product, or an antigen, is called oral tolerance. Research into this mechanism of action has revealed that several distinct types of regulatory T cells facilitate this occurrence by releasing anti-inflammatory cytokines. It has also been shown that this effect is transitory in nature, requiring that the food, or antigen, be consumed on a consistent basis to maintain the tolerogenic state [19]. Given these findings, it is plausible that supplementation with undenatured type II collagen might relieve joint discomfort and restore joint function in healthy participants via an acute immunomodulatory effect.
Exercise can cause pain and stiffness in knee joints which resembles the symptoms of localized inflammation [20]. Knee pain can present as a self-prescribed contraindication to exercise, many older adults living with knee pain possess uncertainty of their ability to exercise safely [6]. Moreover, mechanistic research suggests that intense or repetitive exercise leads to a decline in articular cartilage [21]. Thus, it may be that exercise can induce similar physiological outcomes to those that take place in arthritic disease [21]. While systematic reviews show that exercise is generally safe for adults with knee pain [22], individuals suffering from exercise-induced knee pain may remain wary of their ability to participate in a regimented exercise program. Reducing knee pain onset during exercise, then, is of great interest to this population as the benefits of exercise do not wane in old age [23].
In the United States, approximately 31 million osteoarthritic patients accumulated medical expenses exceeding 340 billion dollars between 2008 and 2011 [24]. Knee osteoarthritis is a prevalent health concern in the general population. In fact, the most frequent occurrence of symptomatic osteoarthritis is the knee joint, which affects nearly one quarter of the general population [25]. In individuals over 50 years old, osteoarthritis was reported to be the most common cause of knee pain [26]. Further, the onset of knee pain has been shown to decrease physical activity and potentially speed age-related muscle loss that is commonly referred to as sarcopenia [27]. Consequently, after the age of 50, individuals experience a steady decline in function that eventually leads to loss of independence [27]. Extensive data has demonstrated that knee pain presents with several psychological comorbidities including, but not limited to, depression, anxiety, and impairments in social interactions [28]. Furthermore, cross-sectional studies have reported a positive relationship between osteoarthritis and cardiovascular and metabolic diseases [29] while a systematic review indicated that the worsening of osteoarthritic pain paired with such diseases leads to greater deterioration in physical functioning [30]. Hence, remedies for osteoarthritic pain not only apply to localized pain, but to general health.
UC-II® collagen is a unique nutritional supplement that has demonstrated support for joint health in humans and animals. In the current report, daily supplementation (40 mg) of undenatured type II collagen for 120 days prompted significant improvements in KOOS survey scores compared to placebo in a cohort of participants aged at least 50 years who experienced exercise-induced knee pain. Indeed, participants in the undenatured type II collagen supplementation group exhibited greater improvements in joint discomfort during standing upright (39.12% vs 0%), ascending stairs (30.8% vs 0%), going up or down stairs (34.5% vs 14.8%), bending to floor/picking up object (13.1% vs -8.7%), and squatting (34.5% vs 0%). Thus, oral consumption of undenatured type II collagen could be an applied treatment to improve knee pain and function.
Ethics approval and consent to participate
The original study was conducted in 2013 according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (Copernicus Group IRB, Cary, NC). Current post hoc analysis data is from the original study 2013. Informed consent was obtained from all subjects involved in the study in 2013.
Consent for publication
All authors have read and agreed to the submitted version of the manuscript.
Availability of data and materials
The data presented in this study are available on request from the corresponding author.
Competing interests
Zainulabedin Saiyed, Shane Durkee, and Vijaya Juturu are employed by Lonza Consumer Health Inc. James Bowman is an independent statistical consultant who ran the analysis for the current manuscript on behalf of Lonza CHI Inc.
Funding
Lonza CHI Inc.
Authors' contributions
Conceptualization: ZS and VJ.; Methodology and data collection: ZS, JB and VJ.; Formal analysis: JB and VJ.; Data curation: ZS., SD and VJ.; Writing, review and editing-original draft preparation, supervision, project administration: ZS and VJ.
Citation: Saiyed Z, Durkee S, Bowman J, Juturu V (2022) Efficacy of UC-II® Undenatured Type II Collagen on Knee Joint Function in Healthy Subjects: An Exploratory Post Hoc Analysis of a Randomized, Double-Blind, Placebo-Controlled Trial. J Clin Trials. S15:002.
Received: 07-Dec-2021 Accepted: 21-Dec-2021 Published: 28-Dec-2021
Copyright: © 2022 Saiyed Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.